

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50290967 1-{(5R,7S,8S)-8-[(2-Benzofuran-4-yl-acetyl)-methyl-amino]-1-oxa-spiro[4.5]dec-7-yl}-pyrrolidinium::2-Benzofuran-4-yl-N-methyl-N-((5R,7S,8S)-7-pyrrolidin-1-yl-1-oxa-spiro[4.5]dec-8-yl)-acetamide::CHEMBL318859::CI-977
SMILES: CN([C@H]1CC[C@@]2(CCCO2)C[C@@H]1N1CCCC1)C(=O)Cc1cccc2occc12
InChI Key: InChIKey=JMBYBVLCYODBJQ-HFMPRLQTSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50290967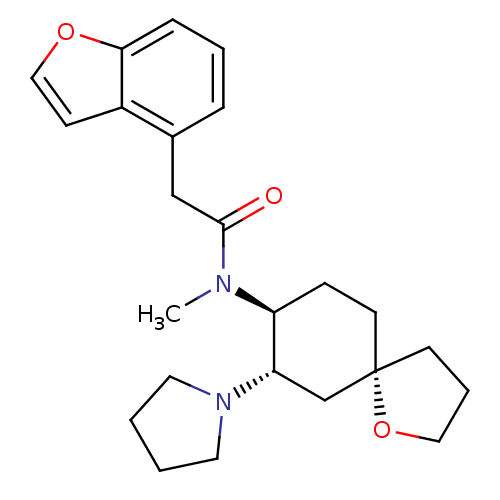 (1-{(5R,7S,8S)-8-[(2-Benzofuran-4-yl-acetyl)-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of binding of [3H]-U-69,593 to cloned rat Opioid receptor kappa 1 expressed in CHO cell line | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50290967 (1-{(5R,7S,8S)-8-[(2-Benzofuran-4-yl-acetyl)-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Opioid receptor kappa 1 in chinese Hamster Ovary (CHO) cell membranes was determined using [3H]U-69593 radioligand | J Med Chem 46: 5162-70 (2003) Article DOI: 10.1021/jm030139v BindingDB Entry DOI: 10.7270/Q2GX4C9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50290967 (1-{(5R,7S,8S)-8-[(2-Benzofuran-4-yl-acetyl)-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Opioid receptor mu 1 in chinese Hamster Ovary (CHO) cells membranes was determined using [3H]-DAMGO radioligand | J Med Chem 46: 5162-70 (2003) Article DOI: 10.1021/jm030139v BindingDB Entry DOI: 10.7270/Q2GX4C9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50290967 (1-{(5R,7S,8S)-8-[(2-Benzofuran-4-yl-acetyl)-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of binding of [3H]-DAGO to rat brain mu opioid receptor | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50290967 (1-{(5R,7S,8S)-8-[(2-Benzofuran-4-yl-acetyl)-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory activity against Opioid receptor delta 1 in chinese Hamster Ovary (CHO) cell membranes was determined using [3H]naltrindole radioligand | J Med Chem 46: 5162-70 (2003) Article DOI: 10.1021/jm030139v BindingDB Entry DOI: 10.7270/Q2GX4C9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50290967 (1-{(5R,7S,8S)-8-[(2-Benzofuran-4-yl-acetyl)-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Opioid receptor kappa 1 as reduced contraction in electrically stimulated rabbit vas deferens | Bioorg Med Chem Lett 7: 291-296 (1997) Article DOI: 10.1016/S0960-894X(96)00615-4 BindingDB Entry DOI: 10.7270/Q2G160VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||