

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50292007 CHEMBL1795064::CHEMBL286828::{{2-[2-(2-{Carboxymethyl-[(5-sulfamoyl-[1,3,4]thiadiazol-2-ylcarbamoyl)-methyl]-amino}-ethoxy)-ethoxy]-ethyl}-[(5-sulfamoyl-[1,3,4]thiadiazol-2-ylcarbamoyl)-methyl]-amino}-acetic acid::{{2-[2-(2-{Carboxymethyl-[(5-sulfamoyl-[1,3,4]thiadiazol-2-ylcarbamoyl)-methyl]-amino}-ethoxy)-ethoxy]-ethyl}-[(5-sulfamoyl-[1,3,4]thiadiazol-2-ylcarbamoyl)-methyl]-amino}-acetic acid; compound with Al complex::{{2-[2-(2-{Carboxymethyl-[(5-sulfamoyl-[1,3,4]thiadiazol-2-ylcarbamoyl)-methyl]-amino}-ethoxy)-ethoxy]-ethyl}-[(5-sulfamoyl-[1,3,4]thiadiazol-2-ylcarbamoyl)-methyl]-amino}-acetic acid; compound with Cu complex::{{2-[2-(2-{Carboxymethyl-[(5-sulfamoyl-[1,3,4]thiadiazol-2-ylcarbamoyl)-methyl]-amino}-ethoxy)-ethoxy]-ethyl}-[(5-sulfamoyl-[1,3,4]thiadiazol-2-ylcarbamoyl)-methyl]-amino}-acetic acid; compound with Zn complex
SMILES: NS(=O)(=O)c1nnc(NC(=O)CN(CCOCCOCCN(CC(O)=O)CC(=O)Nc2nnc(s2)S(N)(=O)=O)CC(O)=O)s1
InChI Key: InChIKey=GBDGTOLMAXSIBD-UHFFFAOYSA-N
Data: 6 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50292007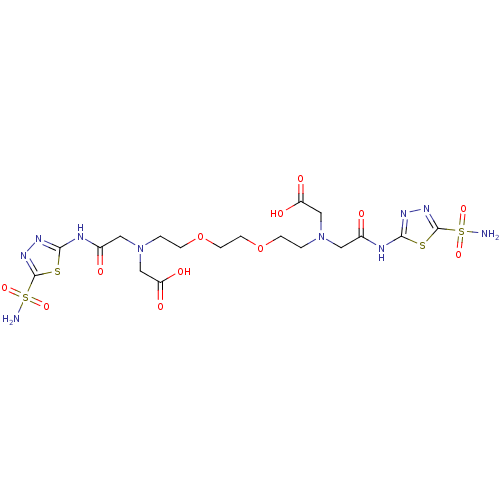 (CHEMBL1795064 | CHEMBL286828 | {{2-[2-(2-{Carboxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against Human carbonic anhydrase II | J Med Chem 45: 1466-76 (2002) BindingDB Entry DOI: 10.7270/Q2N29XN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50292007 (CHEMBL1795064 | CHEMBL286828 | {{2-[2-(2-{Carboxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against Human carbonic anhydrase II | J Med Chem 45: 1466-76 (2002) BindingDB Entry DOI: 10.7270/Q2N29XN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase IV (Bos taurus (bovine)) | BDBM50292007 (CHEMBL1795064 | CHEMBL286828 | {{2-[2-(2-{Carboxym...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV | J Med Chem 45: 1466-76 (2002) BindingDB Entry DOI: 10.7270/Q2N29XN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic Anhydrase IV (Bos taurus (bovine)) | BDBM50292007 (CHEMBL1795064 | CHEMBL286828 | {{2-[2-(2-{Carboxym...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against bovine carbonic anhydrase IV | J Med Chem 45: 1466-76 (2002) BindingDB Entry DOI: 10.7270/Q2N29XN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50292007 (CHEMBL1795064 | CHEMBL286828 | {{2-[2-(2-{Carboxym...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against Human carbonic anhydrase I | J Med Chem 45: 1466-76 (2002) BindingDB Entry DOI: 10.7270/Q2N29XN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50292007 (CHEMBL1795064 | CHEMBL286828 | {{2-[2-(2-{Carboxym...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibitory activity against Human carbonic anhydrase I | J Med Chem 45: 1466-76 (2002) BindingDB Entry DOI: 10.7270/Q2N29XN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||