

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50292332 2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]isoquinolinylium; chloride::2,3,9,10-tetramethoxy-5,6-dihydro-isoquino[3,2-a]isoquinolinylium::CHEMBL1270849::CHEMBL2163795::CHEMBL274189::GNF-Pf-4086::PALMATINE
SMILES: COc1cc2CC[n+]3cc4c(OC)c(OC)ccc4cc3-c2cc1OC
InChI Key: InChIKey=QUCQEUCGKKTEBI-UHFFFAOYSA-N
Data: 11 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM50292332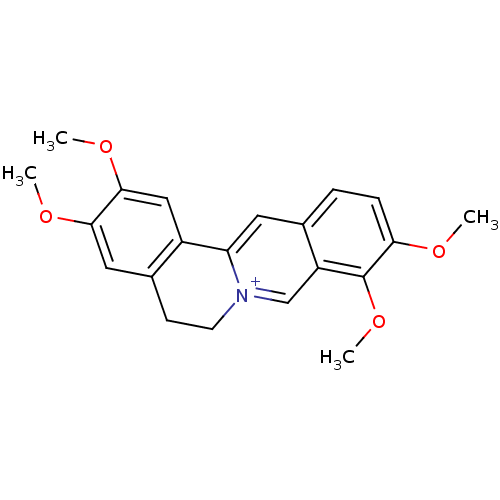 (2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of recombinant Influenza A virus H1N1 neuraminidase using 4-methylumbelliferyl-alpha-D-Nacetylneuraminic acid sodium salt hydrate as subst... | Bioorg Med Chem 22: 6047-52 (2014) Article DOI: 10.1016/j.bmc.2014.09.004 BindingDB Entry DOI: 10.7270/Q269755J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM50292332 (2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus H5N1 neuraminidase using 4-methylumbelliferyl-alpha-D-Nacetylneuraminic acid sodium salt hydrate as substrate by fluo... | Bioorg Med Chem 22: 6047-52 (2014) Article DOI: 10.1016/j.bmc.2014.09.004 BindingDB Entry DOI: 10.7270/Q269755J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (West Nile virus) | BDBM50292332 (2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of KwaZulu-Natal Curated by ChEMBL | Assay Description Non-competitive inhibition of West Nile virus NS2B-NS3 protease | Eur J Med Chem 87: 677-702 (2014) Article DOI: 10.1016/j.ejmech.2014.10.010 BindingDB Entry DOI: 10.7270/Q2WW7K9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50292332 (2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cheng Kung University Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 expressed in Escherichia coli assessed as inhibition of nifedipine oxidation | J Nat Prod 70: 1930-3 (2007) Article DOI: 10.1021/np0704248 BindingDB Entry DOI: 10.7270/Q2JH3KX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50292332 (2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University Curated by ChEMBL | Assay Description Inhibition of human AChE by Ellman's method | Bioorg Med Chem 20: 6669-79 (2012) Article DOI: 10.1016/j.bmc.2012.09.040 BindingDB Entry DOI: 10.7270/Q2ZG6TBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50292332 (2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Snake venom metalloproteinase Bap1 (Bothrops asper) | BDBM50292332 (2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of snake venom BaP1 using Abz-Ala-Gly-Leu-Ala-Nba as substrate incubated for 30 mins prior to substrate addition by fluorescence spectroph... | ACS Med Chem Lett 3: 540-543 (2012) Article DOI: 10.1021/ml300068r BindingDB Entry DOI: 10.7270/Q2542PVS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292332 (2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | J Nat Prod (2010) Article DOI: 10.1021/np100247r BindingDB Entry DOI: 10.7270/Q2HH6M22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50292332 (2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ... | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterases; ACHE & BCHE (Homo sapiens (Human)) | BDBM50292332 (2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate measured for 1 min by Ellman's method | J Nat Prod 82: 239-248 (2019) Article DOI: 10.1021/acs.jnatprod.8b00592 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292332 (2,3,9,10-Tetramethoxy-5,6-dihydro-isoquino[3,2-a]i...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by Ellman's method | Bioorg Med Chem 20: 6669-79 (2012) Article DOI: 10.1016/j.bmc.2012.09.040 BindingDB Entry DOI: 10.7270/Q2ZG6TBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||