

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50292493 CHEMBL452973::{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}({[({[({[4-(2-carbamoyl-2-acetamidoethyl)phenyl]amino}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)(hydroxy)phosphoryl]oxy})phosphinic acid
SMILES: CC(=O)NC(Cc1ccc(NP(O)(=O)OP(O)(=O)OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)cc1)C(N)=O
InChI Key: InChIKey=BJAGKSRQTGUUGO-YDSQADTNSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292493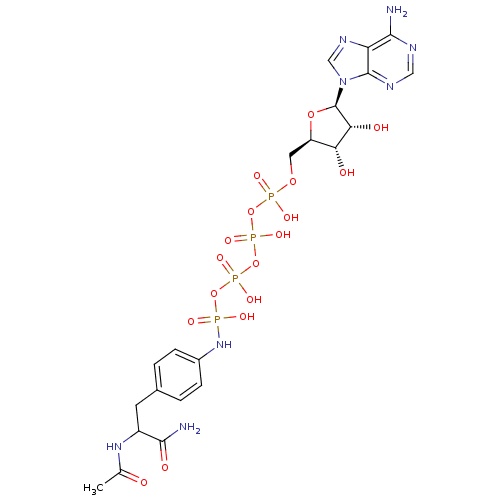 (CHEMBL452973 | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline& French Laboratories Curated by ChEMBL | Assay Description Inhibition of p60v-abl tyrosine kinase from Abelson murine leukemia virus | J Med Chem 31: 1768-72 (1988) BindingDB Entry DOI: 10.7270/Q2833SMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM50292493 (CHEMBL452973 | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Abelson murine leukemia virus p60 v-abl | J Nat Prod 55: 1529-1560 (1992) Article DOI: 10.1021/np50089a001 BindingDB Entry DOI: 10.7270/Q2J966CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||