

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50293855 4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-1H-benzimidazol-1-yl}-1-[(1S,3S,4R)-spiro[bicyclo[2.2.1]heptane-2,1'-cyclopropan]-3-ylmethyl]piperidine hydrochloride::CHEMBL564840
SMILES: OC[C@H](O)Cn1c2ccccc2n(C2CCN(C[C@H]3[C@H]4CC[C@H](C4)C33CC3)CC2)c1=O
InChI Key: InChIKey=DTAPURRKADLRKH-ZSXPUABSSA-N
Data: 11 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50293855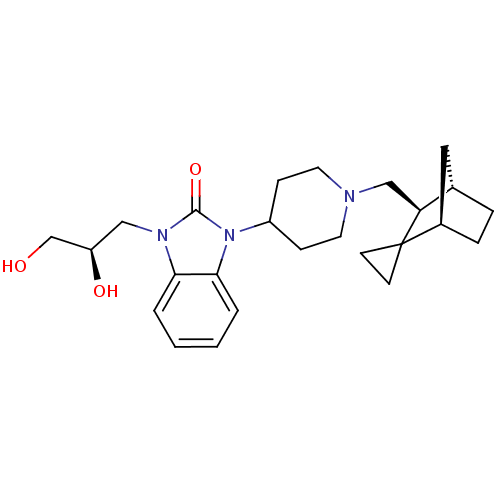 (4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human cloned delta opioid receptor expressed in CHO cells | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50293855 (4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor expressed in CHO cells by [35S]GTPgammaS binding assay | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50293855 (4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes assessed as inhibition of acetophenacetin odeethylation | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50293855 (4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C8 in human liver microsomes assessed as inhibition of paclitaxel 6-alpha-hydroxylation | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50293855 (4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes assessed as inhibition of diclofenac 4'-hydroxylation | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50293855 (4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human cloned kappa opioid receptor expressed in CHO cells | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50293855 (4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes assessed as inhibition of testosterone 6-beta-hydroxylation | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50293855 (4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of radiolabeled MK-499 from human ERG expressed in HEK293 cells coexpressing IKr channel protein | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50293855 (4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human cloned ORL1 receptor expressed in CHO cells | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50293855 (4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human cloned mu opioid receptor expressed in CHO cells | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50293855 (4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes assessed as inhibition of dextrometorphan O-demethylation | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||