

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50294701 4-((2-chlorobenzyl)(1-(5-methyl-1,3,4-oxadiazol-2-yl)ethyl)amino)-2-chlorobenzonitrile::CHEMBL559581
SMILES: CC(N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1)c1nnc(C)o1
InChI Key: InChIKey=QIJJYGUDEMYDMJ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294701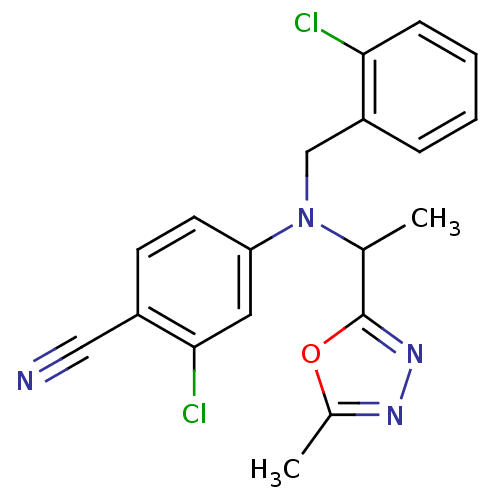 (4-((2-chlorobenzyl)(1-(5-methyl-1,3,4-oxadiazol-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells by alkaline phosphatase release based reporter gene assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294701 (4-((2-chlorobenzyl)(1-(5-methyl-1,3,4-oxadiazol-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor ligand binding domain by fluorimetric assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50294701 (4-((2-chlorobenzyl)(1-(5-methyl-1,3,4-oxadiazol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP2C19 | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||