

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50294835 4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*2,6*]dec-8-en-4-yl)-N-methyl-N-quinolin-8-yl-benzamide::CHEMBL551505::CHEMBL562310::N-(Quinolin-8-yl)-4-(endo-4-aza-3,5-dioxotricyclo[5.2.1.02,6]oct-8-en-4-yl)benzamide, 1
SMILES: Oc1c2[C@H]3C[C@H](C=C3)c2c(O)n1-c1ccc(cc1)C(=O)Nc1cccc2cccnc12
InChI Key: InChIKey=OGYSABKWWQZOIU-CALCHBBNSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein Wnt (Mus musculus (Mouse)) | BDBM50294835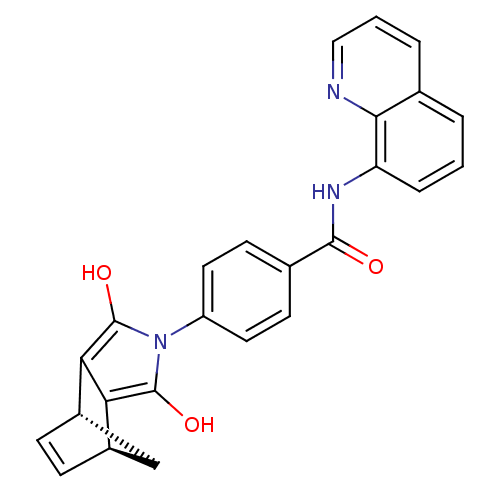 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | 7.4 | 4 |
University of Texas Southwestern Medical Center | Assay Description Inhibition assay of Wnt response treated with exogenously supplied of Wnt protein ('exogenous Wnt' test) | Nat Chem Biol 5: 100-7 (2009) Article DOI: 10.1038/nchembio.137 BindingDB Entry DOI: 10.7270/Q2HX1B20 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal GST-tagged TNKS2 ADP-ART catalytic domain (1001 to 1327 residues) using histone H2A as substrate incubated... | ACS Med Chem Lett 11: 862-868 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of PARP1 (unknown origin) using histone as substrate after 1 hr by luminescence assay | J Med Chem 56: 4320-42 (2013) Article DOI: 10.1021/jm4000038 BindingDB Entry DOI: 10.7270/Q2QF8V74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of PARP2 (unknown origin) using histone as substrate after 1 hr by luminescence assay | J Med Chem 56: 4320-42 (2013) Article DOI: 10.1021/jm4000038 BindingDB Entry DOI: 10.7270/Q2QF8V74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of PARP2 (unknown origin) | J Med Chem 56: 1341-5 (2013) Article DOI: 10.1021/jm301607v BindingDB Entry DOI: 10.7270/Q21N82DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human TNKS1 (1091 to 1325 amino acid residues) after 60 mins by autoparsylation assay | J Med Chem 56: 1341-5 (2013) Article DOI: 10.1021/jm301607v BindingDB Entry DOI: 10.7270/Q21N82DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human TNKS2 (946 to 1162 amino acid residues) after 60 mins by autoparsylation assay | J Med Chem 56: 1341-5 (2013) Article DOI: 10.1021/jm301607v BindingDB Entry DOI: 10.7270/Q21N82DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University Curated by ChEMBL | Assay Description Inhibition of PARP1 | J Med Chem 55: 1360-7 (2012) Article DOI: 10.1021/jm201510p BindingDB Entry DOI: 10.7270/Q2WS8V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University Curated by ChEMBL | Assay Description Inhibition of TNKS1 | J Med Chem 55: 1360-7 (2012) Article DOI: 10.1021/jm201510p BindingDB Entry DOI: 10.7270/Q2WS8V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University Curated by ChEMBL | Assay Description Inhibition of TNKS2 | J Med Chem 55: 1360-7 (2012) Article DOI: 10.1021/jm201510p BindingDB Entry DOI: 10.7270/Q2WS8V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Abo Akademi University Curated by ChEMBL | Assay Description Activity of N-terminus hexaHis-tagged human TNSK2 expressed in Escherichia coli BL21 (DE3) cells using biotinylated NAD+ as substrate after 90 mins b... | J Med Chem 55: 1360-7 (2012) Article DOI: 10.1021/jm201510p BindingDB Entry DOI: 10.7270/Q2WS8V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
Abo Akademi University Curated by ChEMBL | Assay Description Activity of N-terminus hexaHis-tagged human TNSK2 expressed in Escherichia coli BL21 (DE3) cells using biotinylated NAD+ as substrate after 90 mins b... | J Med Chem 55: 1360-7 (2012) Article DOI: 10.1021/jm201510p BindingDB Entry DOI: 10.7270/Q2WS8V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University Curated by ChEMBL | Assay Description Inhibition of human PARP1 using NAD+ as substrate after 90 mins by fluorescence analysis | J Med Chem 55: 1360-7 (2012) Article DOI: 10.1021/jm201510p BindingDB Entry DOI: 10.7270/Q2WS8V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University Curated by ChEMBL | Assay Description Inhibition of human PARP2 using NAD+ as substrate after 90 mins by fluorescence analysis | J Med Chem 55: 1360-7 (2012) Article DOI: 10.1021/jm201510p BindingDB Entry DOI: 10.7270/Q2WS8V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abo Akademi University Curated by ChEMBL | Assay Description Inhibition of PARP2 | J Med Chem 55: 1360-7 (2012) Article DOI: 10.1021/jm201510p BindingDB Entry DOI: 10.7270/Q2WS8V8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 303 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal GST-tagged TNKS1 ADP-ART catalytic domain (1001 to 1327 residues) using histone H2A as substrate incubated... | ACS Med Chem Lett 11: 862-868 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene protein Wnt-3 (Homo sapiens (Human)) | BDBM50294835 (4-((1S,2S,6R,7R)-3,5-Dioxo-4-aza-tricyclo[5.2.1.0*...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
The University of Texas Southwestern Medical Center at Dallas Curated by ChEMBL | Assay Description Inhibition of Wnt3 expressed in mouse L-cells assessed as inhibition of Wnt/catanin signaling pathway by luciferase reporter gene assay | Bioorg Med Chem Lett 19: 3825-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.040 BindingDB Entry DOI: 10.7270/Q2DZ08BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||