

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50295343 (R,R,2S,2'S,4S,4'S,5R,5'R,6R,6'R)-2,2'-(14-(21-((2S,4S,5R,6R)-5-acetamido-2-carboxy-4-hydroxy-6-((1R,2R)-1,2,3-trihydroxypropyl)tetrahydro-2H-pyran-2-ylthio)-8,8-bis(3-(3-(5-((2S,4S,5R,6R)-5-acetamido-2-carboxy-4-hydroxy-6-((1R,2R)-1,2,3-trihydroxypropyl)tetrahydro-2H-pyran-2-ylthio)pentylthio)propoxy)propyl)-4,4-dimethyl-12-oxa-16-thia-4,8-disilahenicosyl)-14-(3-(3-(5-((2S,4S,5R,6R)-5-acetamido-2-carboxy-4-hydroxy-6-((1R,2R)-1,2,3-trihydroxypropyl)tetrahydro-2H-pyran-2-ylthio)pentylthio)pr::CHEMBL553110
SMILES: CC(=O)N[C@@H]1[C@@H](O)C[C@](O[C@H]1[C@H](O)[C@H](O)CO)(SCCCCCSCCCOCCC[Si](CCCOCCCSCCCCCS[C@@]1(C[C@H](O)[C@@H](NC(C)=O)[C@@H](O1)[C@H](O)[C@H](O)CO)C(O)=O)(CCCOCCCSCCCCCS[C@@]1(C[C@H](O)[C@@H](NC(C)=O)[C@@H](O1)[C@H](O)[C@H](O)CO)C(O)=O)CCC[Si](C)(C)CCC[Si](CCCOCCCSCCCCCS[C@@]1(C[C@H](O)[C@@H](NC(C)=O)[C@@H](O1)[C@H](O)[C@H](O)CO)C(O)=O)(CCCOCCCSCCCCCS[C@@]1(C[C@H](O)[C@@H](NC(C)=O)[C@@H](O1)[C@H](O)[C@H](O)CO)C(O)=O)CCCOCCCSCCCCCS[C@@]1(C[C@H](O)[C@@H](NC(C)=O)[C@@H](O1)[C@H](O)[C@H](O)CO)C(O)=O)C(O)=O
InChI Key: InChIKey=NWXGRUCWGDOSDX-FMCZGITMSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza A virus (strain A/Memphis/1/1971 H3N2)) | BDBM50295343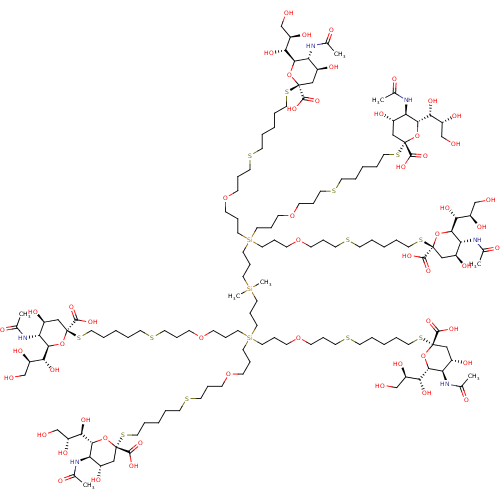 ((R,R,2S,2'S,4S,4'S,5R,5'R,6R,6'R)-2,2'-(14-(21-((2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Saitama University Curated by ChEMBL | Assay Description Inhibition of human influenza A/Memphis/1/71(H3N2) sialidase by fluorescence spectrophotometry | Bioorg Med Chem 17: 5451-64 (2009) Article DOI: 10.1016/j.bmc.2009.06.036 BindingDB Entry DOI: 10.7270/Q26973MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||