

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50295811 12,13-[2-carboxymethyl-cis-2,3-dihydroxy)-1,4-butyl]-6,7,12,13-tetrahyhydro-5,7-dioxo-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazole::CHEMBL564940
SMILES: COC(=O)[C@]1(O)Cn2c3ccccc3c3c4C(=O)NC(=O)c4c4c5ccccc5n(C[C@H]1O)c4c23
InChI Key: InChIKey=DMECWJSRBVZTGX-DXPJPUQTSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50295811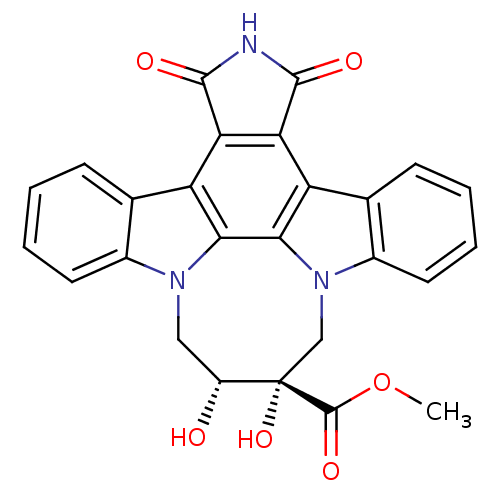 (12,13-[2-carboxymethyl-cis-2,3-dihydroxy)-1,4-buty...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of LCK-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50295811 (12,13-[2-carboxymethyl-cis-2,3-dihydroxy)-1,4-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of JAK3 expressed in insect Sf21 cells assessed as inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 2 (Homo sapiens (Human)) | BDBM50295811 (12,13-[2-carboxymethyl-cis-2,3-dihydroxy)-1,4-buty...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of MK2-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein kinase ZAP-70 (Homo sapiens (Human)) | BDBM50295811 (12,13-[2-carboxymethyl-cis-2,3-dihydroxy)-1,4-buty...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of Zap70-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50295811 (12,13-[2-carboxymethyl-cis-2,3-dihydroxy)-1,4-buty...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development L.L.C. 920 Route 202 Curated by ChEMBL | Assay Description Inhibition of SYK-mediated inhibition of biotinylated substrate phosphorylation | Bioorg Med Chem Lett 19: 3333-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.039 BindingDB Entry DOI: 10.7270/Q2ZK5GQ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||