 Found 5 hits for monomerid = 50296330
Found 5 hits for monomerid = 50296330 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296330
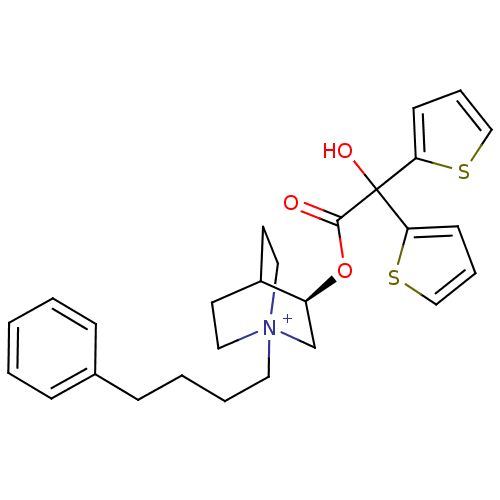
((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(4-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCCc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(30.44,-17.2,;29.36,-18.29,;30.69,-19.06,;30.7,-20.6,;32.02,-18.28,;33.36,-19.05,;33.36,-20.59,;34.69,-21.35,;34.67,-22.89,;36,-23.67,;37.34,-22.92,;38.67,-23.7,;40.01,-22.94,;41.33,-23.73,;42.67,-22.97,;42.69,-21.43,;41.35,-20.65,;40.02,-21.41,;36.02,-20.59,;36.02,-19.05,;34.69,-18.27,;35.11,-19.51,;34.06,-19.86,;29.35,-16.75,;28.1,-15.84,;28.57,-14.38,;30.11,-14.37,;30.59,-15.84,;28.02,-19.06,;26.61,-18.44,;25.58,-19.59,;26.36,-20.92,;27.86,-20.59,)| Show InChI InChI=1S/C27H32NO3S2/c29-26(27(30,24-11-6-18-32-24)25-12-7-19-33-25)31-23-20-28(16-13-22(23)14-17-28)15-5-4-10-21-8-2-1-3-9-21/h1-3,6-9,11-12,18-19,22-23,30H,4-5,10,13-17,20H2/q+1/t22?,23-,28?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ALMIRALL, S.A.
US Patent
| Assay Description
The binding of [3H]-NMS to human muscarinic receptors was performed according to Waelbroek et al (1990) (1). Assays were carried out at 25° C. Membra... |
US Patent US9333195 (2016)
BindingDB Entry DOI: 10.7270/Q2GF0SDB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296330

((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(4-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCCc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(30.44,-17.2,;29.36,-18.29,;30.69,-19.06,;30.7,-20.6,;32.02,-18.28,;33.36,-19.05,;33.36,-20.59,;34.69,-21.35,;34.67,-22.89,;36,-23.67,;37.34,-22.92,;38.67,-23.7,;40.01,-22.94,;41.33,-23.73,;42.67,-22.97,;42.69,-21.43,;41.35,-20.65,;40.02,-21.41,;36.02,-20.59,;36.02,-19.05,;34.69,-18.27,;35.11,-19.51,;34.06,-19.86,;29.35,-16.75,;28.1,-15.84,;28.57,-14.38,;30.11,-14.37,;30.59,-15.84,;28.02,-19.06,;26.61,-18.44,;25.58,-19.59,;26.36,-20.92,;27.86,-20.59,)| Show InChI InChI=1S/C27H32NO3S2/c29-26(27(30,24-11-6-18-32-24)25-12-7-19-33-25)31-23-20-28(16-13-22(23)14-17-28)15-5-4-10-21-8-2-1-3-9-21/h1-3,6-9,11-12,18-19,22-23,30H,4-5,10,13-17,20H2/q+1/t22?,23-,28?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | 25 |
ALMIRALL, S.A.
US Patent
| Assay Description
Assays were carried out at 25° C. Membrane preparations from stably transfected chinese hamster ovary-K1 cells (CHO) expressing the genes for the... |
US Patent US9687478 (2017)
BindingDB Entry DOI: 10.7270/Q2RV0KV9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50296330

((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(4-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCCc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(30.44,-17.2,;29.36,-18.29,;30.69,-19.06,;30.7,-20.6,;32.02,-18.28,;33.36,-19.05,;33.36,-20.59,;34.69,-21.35,;34.67,-22.89,;36,-23.67,;37.34,-22.92,;38.67,-23.7,;40.01,-22.94,;41.33,-23.73,;42.67,-22.97,;42.69,-21.43,;41.35,-20.65,;40.02,-21.41,;36.02,-20.59,;36.02,-19.05,;34.69,-18.27,;35.11,-19.51,;34.06,-19.86,;29.35,-16.75,;28.1,-15.84,;28.57,-14.38,;30.11,-14.37,;30.59,-15.84,;28.02,-19.06,;26.61,-18.44,;25.58,-19.59,;26.36,-20.92,;27.86,-20.59,)| Show InChI InChI=1S/C27H32NO3S2/c29-26(27(30,24-11-6-18-32-24)25-12-7-19-33-25)31-23-20-28(16-13-22(23)14-17-28)15-5-4-10-21-8-2-1-3-9-21/h1-3,6-9,11-12,18-19,22-23,30H,4-5,10,13-17,20H2/q+1/t22?,23-,28?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M3 receptor expressed in CHOK1 cells by microplate scintillation counting |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50296330

((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(4-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCCc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(30.44,-17.2,;29.36,-18.29,;30.69,-19.06,;30.7,-20.6,;32.02,-18.28,;33.36,-19.05,;33.36,-20.59,;34.69,-21.35,;34.67,-22.89,;36,-23.67,;37.34,-22.92,;38.67,-23.7,;40.01,-22.94,;41.33,-23.73,;42.67,-22.97,;42.69,-21.43,;41.35,-20.65,;40.02,-21.41,;36.02,-20.59,;36.02,-19.05,;34.69,-18.27,;35.11,-19.51,;34.06,-19.86,;29.35,-16.75,;28.1,-15.84,;28.57,-14.38,;30.11,-14.37,;30.59,-15.84,;28.02,-19.06,;26.61,-18.44,;25.58,-19.59,;26.36,-20.92,;27.86,-20.59,)| Show InChI InChI=1S/C27H32NO3S2/c29-26(27(30,24-11-6-18-32-24)25-12-7-19-33-25)31-23-20-28(16-13-22(23)14-17-28)15-5-4-10-21-8-2-1-3-9-21/h1-3,6-9,11-12,18-19,22-23,30H,4-5,10,13-17,20H2/q+1/t22?,23-,28?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M2 receptor expressed in CHOK1 cells by microplate scintillation counting |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50296330

((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(4-phe...)Show SMILES OC(C(=O)O[C@H]1C[N+]2(CCCCc3ccccc3)CCC1CC2)(c1cccs1)c1cccs1 |r,wD:5.4,(30.44,-17.2,;29.36,-18.29,;30.69,-19.06,;30.7,-20.6,;32.02,-18.28,;33.36,-19.05,;33.36,-20.59,;34.69,-21.35,;34.67,-22.89,;36,-23.67,;37.34,-22.92,;38.67,-23.7,;40.01,-22.94,;41.33,-23.73,;42.67,-22.97,;42.69,-21.43,;41.35,-20.65,;40.02,-21.41,;36.02,-20.59,;36.02,-19.05,;34.69,-18.27,;35.11,-19.51,;34.06,-19.86,;29.35,-16.75,;28.1,-15.84,;28.57,-14.38,;30.11,-14.37,;30.59,-15.84,;28.02,-19.06,;26.61,-18.44,;25.58,-19.59,;26.36,-20.92,;27.86,-20.59,)| Show InChI InChI=1S/C27H32NO3S2/c29-26(27(30,24-11-6-18-32-24)25-12-7-19-33-25)31-23-20-28(16-13-22(23)14-17-28)15-5-4-10-21-8-2-1-3-9-21/h1-3,6-9,11-12,18-19,22-23,30H,4-5,10,13-17,20H2/q+1/t22?,23-,28?/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human muscarinic M1 receptor expressed in CHOK1 cells by microplate scintillation counting |
J Med Chem 52: 5076-92 (2010)
Article DOI: 10.1021/jm900132z
BindingDB Entry DOI: 10.7270/Q2SX6F5Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data




