 Found 7 hits for monomerid = 50297825
Found 7 hits for monomerid = 50297825 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50297825
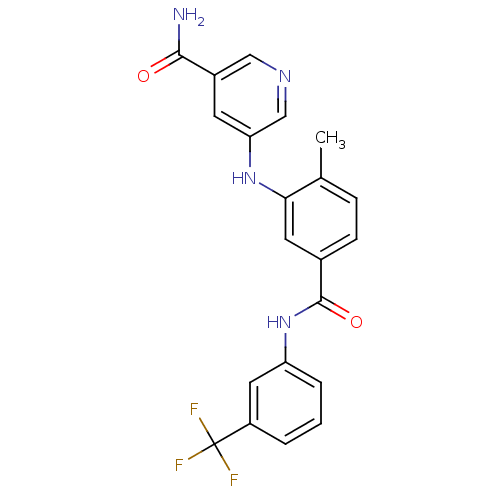
(5-(2-methyl-5-(3-(trifluoromethyl)phenylcarbamoyl)...)Show SMILES Cc1ccc(cc1Nc1cncc(c1)C(N)=O)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C21H17F3N4O2/c1-12-5-6-13(8-18(12)27-17-7-14(19(25)29)10-26-11-17)20(30)28-16-4-2-3-15(9-16)21(22,23)24/h2-11,27H,1H3,(H2,25,29)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
The enzymatic activities DDR2 were tested in LanthaScreen binding assays. The protocol is available from Life Technologies. |
ACS Chem Biol 10: 2687-96 (2015)
Article DOI: 10.1021/acschembio.5b00655
BindingDB Entry DOI: 10.7270/Q2SX6BZ1 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50297825

(5-(2-methyl-5-(3-(trifluoromethyl)phenylcarbamoyl)...)Show SMILES Cc1ccc(cc1Nc1cncc(c1)C(N)=O)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C21H17F3N4O2/c1-12-5-6-13(8-18(12)27-17-7-14(19(25)29)10-26-11-17)20(30)28-16-4-2-3-15(9-16)21(22,23)24/h2-11,27H,1H3,(H2,25,29)(H,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 753 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute
| Assay Description
The enzymatic activities DDR2 were tested in LanthaScreen binding assays. The protocol is available from Life Technologies. |
ACS Chem Biol 10: 2687-96 (2015)
Article DOI: 10.1021/acschembio.5b00655
BindingDB Entry DOI: 10.7270/Q2SX6BZ1 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50297825

(5-(2-methyl-5-(3-(trifluoromethyl)phenylcarbamoyl)...)Show SMILES Cc1ccc(cc1Nc1cncc(c1)C(N)=O)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C21H17F3N4O2/c1-12-5-6-13(8-18(12)27-17-7-14(19(25)29)10-26-11-17)20(30)28-16-4-2-3-15(9-16)21(22,23)24/h2-11,27H,1H3,(H2,25,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of EphB2 |
Bioorg Med Chem Lett 19: 4467-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.029
BindingDB Entry DOI: 10.7270/Q2V69JN0 |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM50297825

(5-(2-methyl-5-(3-(trifluoromethyl)phenylcarbamoyl)...)Show SMILES Cc1ccc(cc1Nc1cncc(c1)C(N)=O)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C21H17F3N4O2/c1-12-5-6-13(8-18(12)27-17-7-14(19(25)29)10-26-11-17)20(30)28-16-4-2-3-15(9-16)21(22,23)24/h2-11,27H,1H3,(H2,25,29)(H,28,30) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged DDR1 in human HEK293 cells assessed as reduction in collagen-1-induced DDR1 phosphorylation incubated for 18 hrs by ELISA |
ACS Med Chem Lett 11: 29-33 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00382 |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50297825

(5-(2-methyl-5-(3-(trifluoromethyl)phenylcarbamoyl)...)Show SMILES Cc1ccc(cc1Nc1cncc(c1)C(N)=O)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C21H17F3N4O2/c1-12-5-6-13(8-18(12)27-17-7-14(19(25)29)10-26-11-17)20(30)28-16-4-2-3-15(9-16)21(22,23)24/h2-11,27H,1H3,(H2,25,29)(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of kinase tracer 178 binding to recombinant human GST-tagged DDR2 (427-855 residues) expressed in baculovirus expression system incubated ... |
ACS Med Chem Lett 11: 29-33 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00382 |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM50297825

(5-(2-methyl-5-(3-(trifluoromethyl)phenylcarbamoyl)...)Show SMILES Cc1ccc(cc1Nc1cncc(c1)C(N)=O)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C21H17F3N4O2/c1-12-5-6-13(8-18(12)27-17-7-14(19(25)29)10-26-11-17)20(30)28-16-4-2-3-15(9-16)21(22,23)24/h2-11,27H,1H3,(H2,25,29)(H,28,30) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of kinase tracer 178 binding to recombinant human GST-tagged DDR1 expressed in baculovirus expression system incubated for 60 mins by TR-F... |
ACS Med Chem Lett 11: 29-33 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00382 |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 2
(Homo sapiens (Human)) | BDBM50297825

(5-(2-methyl-5-(3-(trifluoromethyl)phenylcarbamoyl)...)Show SMILES Cc1ccc(cc1Nc1cncc(c1)C(N)=O)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C21H17F3N4O2/c1-12-5-6-13(8-18(12)27-17-7-14(19(25)29)10-26-11-17)20(30)28-16-4-2-3-15(9-16)21(22,23)24/h2-11,27H,1H3,(H2,25,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of ephrinB1-induced autophosphorylation of EphB2 expressed in human U87 cells after 1 hr by western blot analysis |
Bioorg Med Chem Lett 19: 4467-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.029
BindingDB Entry DOI: 10.7270/Q2V69JN0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data