

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50299053 CHEMBL573714::N-Hydroxy-2-(2-(4-methoxyphenylsulfonyl)phenyl)acetamide
SMILES: COc1ccc(cc1)S(=O)(=O)c1ccccc1CC(=O)NO
InChI Key: InChIKey=ITWGWXCEGHFMHT-UHFFFAOYSA-N
Data: 15 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50299053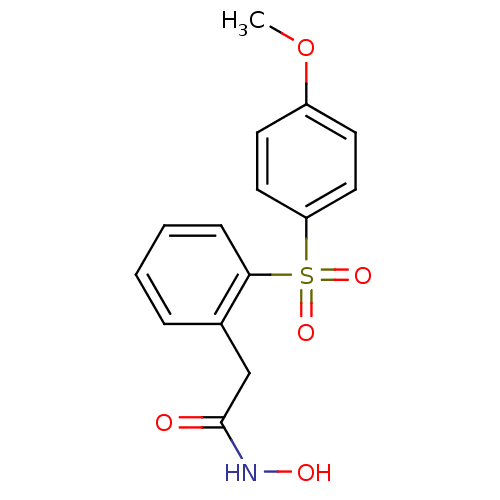 (CHEMBL573714 | N-Hydroxy-2-(2-(4-methoxyphenylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of para-aminophenylmercuric acetate-activated human recombinant pro-MMP9 catalytic domain after 4 hrs by fluorimetry | J Med Chem 52: 6347-61 (2009) Article DOI: 10.1021/jm900335a BindingDB Entry DOI: 10.7270/Q2HQ3ZZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50299053 (CHEMBL573714 | N-Hydroxy-2-(2-(4-methoxyphenylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of para-aminophenylmercuric acetate-activated human pro-MMP1 after 4 hrs by fluorimetry | J Med Chem 52: 6347-61 (2009) Article DOI: 10.1021/jm900335a BindingDB Entry DOI: 10.7270/Q2HQ3ZZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50299053 (CHEMBL573714 | N-Hydroxy-2-(2-(4-methoxyphenylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
UiT The Arctic University of Norway Curated by ChEMBL | Assay Description Inhibition of recombinant human MMP9 after 2 hrs using FS-6 as substrate by fluorometry | Eur J Med Chem 108: 141-53 (2016) BindingDB Entry DOI: 10.7270/Q2HT2R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50299053 (CHEMBL573714 | N-Hydroxy-2-(2-(4-methoxyphenylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
UiT The Arctic University of Norway Curated by ChEMBL | Assay Description Inhibition of recombinant human MMP2 after 2 hrs using FS-6 as substrate by fluorometry | Eur J Med Chem 108: 141-53 (2016) BindingDB Entry DOI: 10.7270/Q2HT2R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pseudolysin (Pseudomonas aeruginosa) | BDBM50299053 (CHEMBL573714 | N-Hydroxy-2-(2-(4-methoxyphenylsulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UiT The Arctic University of Norway Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus pseudolysin pretreated with compound for 15 mins followed by the addition of Abz-Ala-Gly-leu-Ala-p-nitrobe... | Eur J Med Chem 108: 141-53 (2016) BindingDB Entry DOI: 10.7270/Q2HT2R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pseudolysin (Pseudomonas aeruginosa) | BDBM50299053 (CHEMBL573714 | N-Hydroxy-2-(2-(4-methoxyphenylsulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UiT The Arctic University of Norway Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa pseudolysin pretreated with compound for 1 hr followed by the addition of 1.73 uM bradykinin like substrate by s... | Eur J Med Chem 108: 141-53 (2016) BindingDB Entry DOI: 10.7270/Q2HT2R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50299053 (CHEMBL573714 | N-Hydroxy-2-(2-(4-methoxyphenylsulf...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
UiT The Arctic University of Norway Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin pretreated with compound for 1 hr followed by the addition of 3.33 uM bradykinin like substrat... | Eur J Med Chem 108: 141-53 (2016) BindingDB Entry DOI: 10.7270/Q2HT2R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50299053 (CHEMBL573714 | N-Hydroxy-2-(2-(4-methoxyphenylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UiT The Arctic University of Norway Curated by ChEMBL | Assay Description Inhibition of recombinant human ADAM17 after 30 mins using FS-6 as substrate by fluorometry | Eur J Med Chem 108: 141-53 (2016) BindingDB Entry DOI: 10.7270/Q2HT2R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-12 (MMP12) (Homo sapiens (Human)) | BDBM50299053 (CHEMBL573714 | N-Hydroxy-2-(2-(4-methoxyphenylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of autoactivated human pro-MMP12 after 4 hrs by fluorimetry | J Med Chem 52: 6347-61 (2009) Article DOI: 10.1021/jm900335a BindingDB Entry DOI: 10.7270/Q2HQ3ZZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50299053 (CHEMBL573714 | N-Hydroxy-2-(2-(4-methoxyphenylsulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of para-aminophenylmercuric acetate-activated human pro-MMP13 after 4 hrs by fluorimetry | J Med Chem 52: 6347-61 (2009) Article DOI: 10.1021/jm900335a BindingDB Entry DOI: 10.7270/Q2HQ3ZZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase 16 (Homo sapiens (Human)) | BDBM50299053 (CHEMBL573714 | N-Hydroxy-2-(2-(4-methoxyphenylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of human recombinant pro-MMP16 catalytic domain after 4 hrs by fluorimetry | J Med Chem 52: 6347-61 (2009) Article DOI: 10.1021/jm900335a BindingDB Entry DOI: 10.7270/Q2HQ3ZZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50299053 (CHEMBL573714 | N-Hydroxy-2-(2-(4-methoxyphenylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of para-aminophenylmercuric acetate-activated human recombinant pro-MMP2 catalytic domain after 4 hrs by fluorimetry | J Med Chem 52: 6347-61 (2009) Article DOI: 10.1021/jm900335a BindingDB Entry DOI: 10.7270/Q2HQ3ZZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50299053 (CHEMBL573714 | N-Hydroxy-2-(2-(4-methoxyphenylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of para-aminophenylmercuric acetate-activated human pro-MMP8 after 4 hrs by fluorimetry | J Med Chem 52: 6347-61 (2009) Article DOI: 10.1021/jm900335a BindingDB Entry DOI: 10.7270/Q2HQ3ZZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50299053 (CHEMBL573714 | N-Hydroxy-2-(2-(4-methoxyphenylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of trypsin activated human pro-MMP3 after 4 hrs by fluorimetry | J Med Chem 52: 6347-61 (2009) Article DOI: 10.1021/jm900335a BindingDB Entry DOI: 10.7270/Q2HQ3ZZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (MMP14) (Homo sapiens (Human)) | BDBM50299053 (CHEMBL573714 | N-Hydroxy-2-(2-(4-methoxyphenylsulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Inhibition of human recombinant pro-MMP14 catalytic domain after 4 hrs by fluorimetry | J Med Chem 52: 6347-61 (2009) Article DOI: 10.1021/jm900335a BindingDB Entry DOI: 10.7270/Q2HQ3ZZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||