

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CCN(CC)c1nc(NC2CCN(C)CC2)c2cc(OC)c(OC)cc2n1
InChI Key: InChIKey=YMSMSNAIHUSOEZ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50300033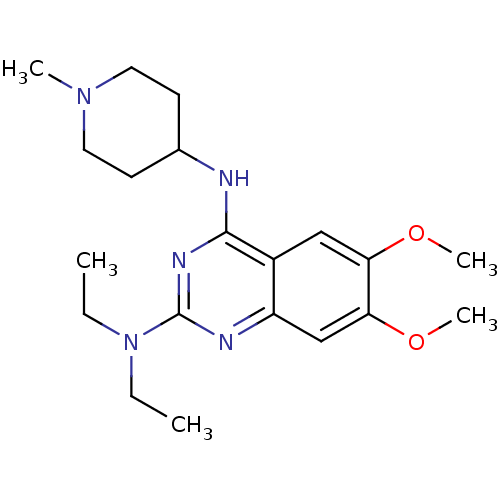 (CHEMBL585180 | N2,N2-diethyl-6,7-dimethoxy-N4-(1-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of G9a by Thioglo assay | J Med Chem 52: 7950-3 (2009) Article DOI: 10.1021/jm901543m BindingDB Entry DOI: 10.7270/Q2542NP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50300033 (CHEMBL585180 | N2,N2-diethyl-6,7-dimethoxy-N4-(1-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of methyl transferase activity of G9a assessed as inhibition of H3K9 methylation by chemiluminescence based oxygen tunneling assay | J Med Chem 53: 5844-57 (2010) Article DOI: 10.1021/jm100478y BindingDB Entry DOI: 10.7270/Q2DZ08H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50300033 (CHEMBL585180 | N2,N2-diethyl-6,7-dimethoxy-N4-(1-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 343 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human G9a catalytic domain (913 to 1193 residues) expressed in Escherichia coli BL21 (DE3) using biotinylated H3 (1 to 25 residues) as ... | Bioorg Med Chem 25: 4414-4423 (2017) Article DOI: 10.1016/j.bmc.2017.06.021 BindingDB Entry DOI: 10.7270/Q2X350ZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50300033 (CHEMBL585180 | N2,N2-diethyl-6,7-dimethoxy-N4-(1-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Activity at methyl transferase activity G9a by enzyme coupled S-adenocylehomocystein detection assay | J Med Chem 53: 5844-57 (2010) Article DOI: 10.1021/jm100478y BindingDB Entry DOI: 10.7270/Q2DZ08H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT1 (Homo sapiens (Human)) | BDBM50300033 (CHEMBL585180 | N2,N2-diethyl-6,7-dimethoxy-N4-(1-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human GLP catalytic domain (951 to 1235 residues) expressed in Escherichia coli BL21 (DE3) using biotinylated H3 (1 to 25 residues) as ... | Bioorg Med Chem 25: 4414-4423 (2017) Article DOI: 10.1016/j.bmc.2017.06.021 BindingDB Entry DOI: 10.7270/Q2X350ZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50300033 (CHEMBL585180 | N2,N2-diethyl-6,7-dimethoxy-N4-(1-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of G9a by Alpha screen assay | J Med Chem 52: 7950-3 (2009) Article DOI: 10.1021/jm901543m BindingDB Entry DOI: 10.7270/Q2542NP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||