

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50302881 CHEMBL570746::N-((3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl)-2'-oxo-1',2'-dihydrospiro[piperidine-4,4'-pyrido[2,3-d][1,3]oxazine]-1-carboxamide
SMILES: Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC4(CC3)OC(=O)Nc3ncccc43)C(=O)N(CC(F)(F)F)C2)c1F
InChI Key: InChIKey=RCACDZUECGFLCL-DNVCBOLYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50302881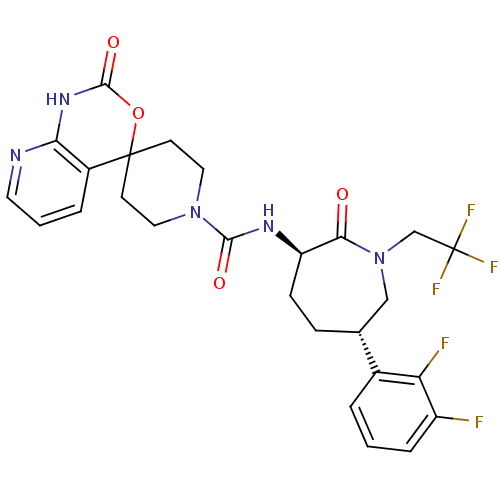 (CHEMBL570746 | N-((3R,6S)-6-(2,3-difluorophenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]human CGRP from human CLR expressed in HEK 293 cells coexpressing human RAMP1 after 3 hrs by scintillation counting | Bioorg Med Chem Lett 19: 6368-72 (2009) Article DOI: 10.1016/j.bmcl.2009.09.066 BindingDB Entry DOI: 10.7270/Q2PC32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50302881 (CHEMBL570746 | N-((3R,6S)-6-(2,3-difluorophenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human CLR expressed in HEK293 cells coexpressing human RAMP1 assessed as inhibition of human CGRPalpha-induced cAMP production... | Bioorg Med Chem Lett 19: 6368-72 (2009) Article DOI: 10.1016/j.bmcl.2009.09.066 BindingDB Entry DOI: 10.7270/Q2PC32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50302881 (CHEMBL570746 | N-((3R,6S)-6-(2,3-difluorophenyl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human CLR expressed in HEK293 cells coexpressing human RAMP1 assessed as inhibition of human CGRPalpha-induced cAMP production... | Bioorg Med Chem Lett 19: 6368-72 (2009) Article DOI: 10.1016/j.bmcl.2009.09.066 BindingDB Entry DOI: 10.7270/Q2PC32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||