

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50303448 Beta-elemonic acid::CHEMBL566929
SMILES: [#6]\[#6](-[#6])=[#6]\[#6]-[#6]-[#6@@H](-[#6@H]1-[#6]-[#6][C@]2([#6])[#6]-3=[#6](-[#6]-[#6][C@@]12[#6])[C@@]1([#6])[#6]-[#6]-[#6](=O)C([#6])([#6])[#6@H]1-[#6]-[#6]-3)-[#6](-[#8])=O
InChI Key: InChIKey=XLPAINGDLCDYQV-DXXDIINSSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase (cyclooxygenase) (Ovis aries (Sheep)) | BDBM50303448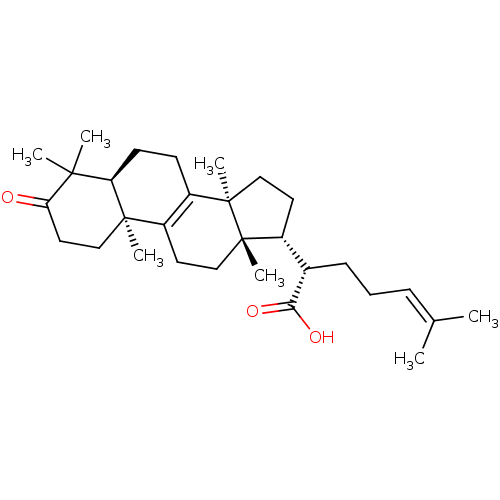 (Beta-elemonic acid | CHEMBL566929) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 mins by HPLC an... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM50303448 (Beta-elemonic acid | CHEMBL566929) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant COX-2 using arachidonic acid as substrate preincubated for 5 mins followed by substrate addition measured after 5 min... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled bile acid receptor 1 (Homo sapiens (Human)) | BDBM50303448 (Beta-elemonic acid | CHEMBL566929) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Universite Louis Pasteur Curated by ChEMBL | Assay Description Agonist activity at TGR5 expressed in CHO cells by CRE-driven luciferase reporter gene assay | J Med Chem 53: 178-90 (2010) Article DOI: 10.1021/jm900872z BindingDB Entry DOI: 10.7270/Q2RJ4KDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50303448 (Beta-elemonic acid | CHEMBL566929) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of prolyl endopeptidase | J Nat Prod 68: 189-93 (2005) Article DOI: 10.1021/np040142x BindingDB Entry DOI: 10.7270/Q22V2H18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50303448 (Beta-elemonic acid | CHEMBL566929) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tuebingen Curated by ChEMBL | Assay Description Inhibition of microsomal PGES1 isolated from IL-1beta-stimulated human A549 cells preincubated for 15 mins followed by substrate addition measured af... | J Nat Prod 77: 1445-51 (2014) Article DOI: 10.1021/np500198g BindingDB Entry DOI: 10.7270/Q23X887B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||