

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50303585 1-(3-tert-butyl-1-(3-nitrophenyl)-1H-pyrazol-5-yl)-3-(4-chlorophenyl)urea::CHEMBL583263
SMILES: CC(C)(C)c1cc(NC(=O)Nc2ccc(Cl)cc2)n(n1)-c1cccc(c1)[N+]([O-])=O
InChI Key: InChIKey=QMJQHTTXRJIVJD-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Discoidin domain-containing receptor 2 (Homo sapiens (Human)) | BDBM50303585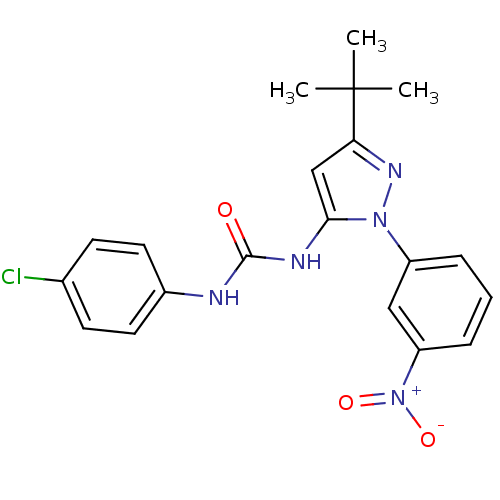 (1-(3-tert-butyl-1-(3-nitrophenyl)-1H-pyrazol-5-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 856 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund Curated by ChEMBL | Assay Description Inhibition of wild type DDR2 (unknown origin) preincubated for 30 mins before substrate addition by FRET assay | J Med Chem 57: 4252-62 (2014) Article DOI: 10.1021/jm500167q BindingDB Entry DOI: 10.7270/Q2Z039P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50303585 (1-(3-tert-butyl-1-(3-nitrophenyl)-1H-pyrazol-5-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society Curated by ChEMBL | Assay Description Displacemnt of N,N'-(2,2'-(3,3'-disulfanediylbis(2,5-dioxopyrrolidine-3,1-diyl))bis(ethane-2,1-diyl))bis(2-(3-(3-tert-butyl-5-(3-naphthalen-1-ylureid... | J Med Chem 53: 357-67 (2010) Article DOI: 10.1021/jm901297e BindingDB Entry DOI: 10.7270/Q29W0FKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50303585 (1-(3-tert-butyl-1-(3-nitrophenyl)-1H-pyrazol-5-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society Curated by ChEMBL | Assay Description Inhibition of p38alpha active form expressed in Escherichia coli BL21(DE3) cells by HTRF assay | J Med Chem 53: 357-67 (2010) Article DOI: 10.1021/jm901297e BindingDB Entry DOI: 10.7270/Q29W0FKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Discoidin domain-containing receptor 2 (Homo sapiens (Human)) | BDBM50303585 (1-(3-tert-butyl-1-(3-nitrophenyl)-1H-pyrazol-5-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund Curated by ChEMBL | Assay Description Binding affinity to human acrylodan-labeled N-terminal His-tagged DDR2 (558 to 855 aa) by FLiK assay | J Med Chem 57: 4252-62 (2014) Article DOI: 10.1021/jm500167q BindingDB Entry DOI: 10.7270/Q2Z039P4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||