

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50303661 4,5-Dichlorothiophene-2-sulfonic Acid[(E)-3-(5-Fluoro-3-methyl-1-naphthalen-2-ylmethyl-1H-indol-7-yl)acryloyl]amide::CHEMBL565799
SMILES: Cc1cn(Cc2ccc3ccccc3c2)c2c(\C=C\C(=O)NS(=O)(=O)c3cc(Cl)c(Cl)s3)cc(F)cc12
InChI Key: InChIKey=HIZNWXUSWJBZEM-CMDGGOBGSA-N
Data: 8 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostanoid EP3 Receptor (Mus musculus (Mouse)) | BDBM50303661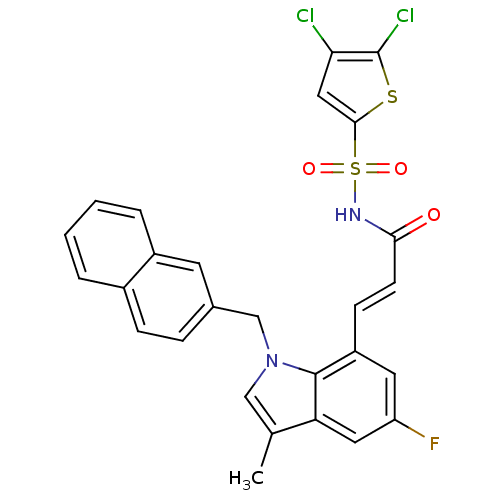 (4,5-Dichlorothiophene-2-sulfonic Acid[(E)-3-(5-Flu...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor after 1 hr by liquid scintillation counting | J Med Chem 53: 18-36 (2010) Article DOI: 10.1021/jm9005912 BindingDB Entry DOI: 10.7270/Q2XP752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid IP receptor (Homo sapiens (Human)) | BDBM50303661 (4,5-Dichlorothiophene-2-sulfonic Acid[(E)-3-(5-Flu...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]iloprost from human IP receptor after 1 hr by liquid scintillation counting | J Med Chem 53: 18-36 (2010) Article DOI: 10.1021/jm9005912 BindingDB Entry DOI: 10.7270/Q2XP752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50303661 (4,5-Dichlorothiophene-2-sulfonic Acid[(E)-3-(5-Flu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor after 1 hr by liquid scintillation counting | J Med Chem 53: 18-36 (2010) Article DOI: 10.1021/jm9005912 BindingDB Entry DOI: 10.7270/Q2XP752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor (Homo sapiens (Human)) | BDBM50303661 (4,5-Dichlorothiophene-2-sulfonic Acid[(E)-3-(5-Flu...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP3 receptor after 1 hr by liquid scintillation counting | J Med Chem 53: 18-36 (2010) Article DOI: 10.1021/jm9005912 BindingDB Entry DOI: 10.7270/Q2XP752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (EP1) (Homo sapiens (Human)) | BDBM50303661 (4,5-Dichlorothiophene-2-sulfonic Acid[(E)-3-(5-Flu...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP1 receptor after 1 hr by liquid scintillation counting | J Med Chem 53: 18-36 (2010) Article DOI: 10.1021/jm9005912 BindingDB Entry DOI: 10.7270/Q2XP752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor (Homo sapiens (Human)) | BDBM50303661 (4,5-Dichlorothiophene-2-sulfonic Acid[(E)-3-(5-Flu...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP3 receptor after 60 mins repeated washing by liquid scintillation counting | J Med Chem 53: 18-36 (2010) Article DOI: 10.1021/jm9005912 BindingDB Entry DOI: 10.7270/Q2XP752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor (Homo sapiens (Human)) | BDBM50303661 (4,5-Dichlorothiophene-2-sulfonic Acid[(E)-3-(5-Flu...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP3 receptor after 1 hr by liquid scintillation counting in presence of 10% human serum | J Med Chem 53: 18-36 (2010) Article DOI: 10.1021/jm9005912 BindingDB Entry DOI: 10.7270/Q2XP752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50303661 (4,5-Dichlorothiophene-2-sulfonic Acid[(E)-3-(5-Flu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP2 receptor after 1 hr by liquid scintillation counting | J Med Chem 53: 18-36 (2010) Article DOI: 10.1021/jm9005912 BindingDB Entry DOI: 10.7270/Q2XP752H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||