

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC(=O)c1ccc2N[C@H]([C@H]3CC=C[C@H]3c2c1)c1cc2OCOc2cc1Br
InChI Key: InChIKey=VHSVKVWHYFBIFJ-HKZYLEAXSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Mus musculus) | BDBM50303803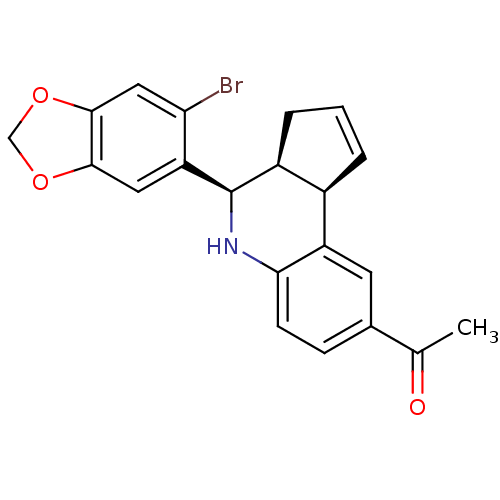 (1-((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico Health Sciences Center Curated by ChEMBL | Assay Description Binding affinity to ERalpha receptor in mouse COS7 cells by competitive binding assay | Nat Chem Biol 5: 421-7 (2009) Article DOI: 10.1038/nchembio.168 BindingDB Entry DOI: 10.7270/Q2RB74TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled estrogen receptor 1 (Homo sapiens (Human)) | BDBM50303803 (1-((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico Health Sciences Center Curated by ChEMBL | Assay Description Displacement of E2-Alexa633 from GFP-tagged GPR30 expressed in COS7 cells by FACS | Nat Chem Biol 2: 207-12 (2006) Article DOI: 10.1038/nchembio775 BindingDB Entry DOI: 10.7270/Q2ZK5GV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled estrogen receptor 1 (Homo sapiens (Human)) | BDBM50303803 (1-((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pittsburgh Chemical Diversity Center (UP-CDC) Curated by ChEMBL | Assay Description Inhibition of estrogen binding to GPR30 (unknown origin) | J Med Chem 56: 7161-76 (2013) Article DOI: 10.1021/jm400132d BindingDB Entry DOI: 10.7270/Q2V69M09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50303803 (1-((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico Health Sciences Center Curated by ChEMBL | Assay Description Displacement of E2-Alexa633 from GFP-tagged ERalpha expressed in COS7 cells by FACS | Nat Chem Biol 2: 207-12 (2006) Article DOI: 10.1038/nchembio775 BindingDB Entry DOI: 10.7270/Q2ZK5GV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled estrogen receptor 1 (Homo sapiens (Human)) | BDBM50303803 (1-((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
New Mexico State University Curated by ChEMBL | Assay Description Binding affinity to GPR30 | J Med Chem 53: 1004-14 (2010) Article DOI: 10.1021/jm9011802 BindingDB Entry DOI: 10.7270/Q2571C3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled estrogen receptor 1 (Homo sapiens (Human)) | BDBM50303803 (1-((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a |
Saint Louis University Curated by ChEMBL | Assay Description Agonist activity at GPER (unknown origin) expressed in human HL60 cells assessed as increase in cAMP accumulation in presence of IBMX after 15 mins b... | ACS Med Chem Lett 9: 901-906 (2018) Article DOI: 10.1021/acsmedchemlett.8b00212 BindingDB Entry DOI: 10.7270/Q2930WTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled estrogen receptor 1 (Homo sapiens (Human)) | BDBM50303803 (1-((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
University of New Mexico Health Sciences Center Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged GPR30 expressed in COS7 cells assessed as half life for increase in intracellular calcium level by spectrofluorimetry | Nat Chem Biol 2: 207-12 (2006) Article DOI: 10.1038/nchembio775 BindingDB Entry DOI: 10.7270/Q2ZK5GV2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50303803 (1-((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | n/a | 184 | n/a | n/a | n/a | n/a |
NMMLSC Curated by PubChem BioAssay | Assay Description University of New Mexico Assay Overview: Assay Support: 1X01 MH077627-01 Assay for Ligands of GPR30 and Classical Estrogen Receptors PI: Eric Prossni... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2VQ313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50303803 (1-((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | n/a | 48.7 | n/a | n/a | n/a | n/a |
NMMLSC Curated by PubChem BioAssay | Assay Description University of New Mexico Assay Overview: Assay Support: 1X01 MH077627-01 Assay for Ligands of GPR30 and Classical Estrogen Receptors PI: Eric Prossni... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2HQ3XB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled estrogen receptor 1 (Homo sapiens (Human)) | BDBM50303803 (1-((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
University of Pittsburgh Chemical Diversity Center (UP-CDC) Curated by ChEMBL | Assay Description Agonist activity at GPR30 (unknown origin) by calcium mobilization assay | J Med Chem 56: 7161-76 (2013) Article DOI: 10.1021/jm400132d BindingDB Entry DOI: 10.7270/Q2V69M09 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POsterior Segregation (Caenorhabditis elegans) | BDBM50303803 (1-((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Broad Institute: MLPCN maternal gene expression Project ID: 2024 Keywords: Zinc finger, C. elegans, maternal gene expression, RNA-protein interac... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q28G8J4C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Zinc finger protein mex-5 (Caenorhabditis elegans) | BDBM50303803 (1-((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a |
Broad Institute Curated by PubChem BioAssay | Assay Description Broad Institute: MLPCN maternal gene expression Project ID: 2024 Keywords: Zinc finger, C. elegans, maternal gene expression, RNA-protein interac... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2D798VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||