

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50303812 (S)-1-(5-Chlorothiophen-2-yl)-1-((5aR,6S)-1-(4-fluorophenyl)-5a-methyl-5,5a,6,7,8,9-hexahydroimidazo[1,5-b]isoquinolin-6-yl)-ethanol::CHEMBL572330
SMILES: C[C@](O)([C@H]1CCCC2=Cc3c(ncn3C[C@]12C)-c1ccc(F)cc1)c1ccc(Cl)s1
InChI Key: InChIKey=FBHBBILQSLGKPC-IGKWTDBASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303812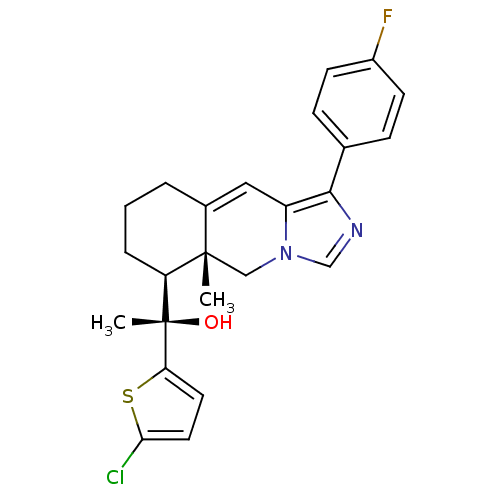 ((S)-1-(5-Chlorothiophen-2-yl)-1-((5aR,6S)-1-(4-flu...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of GS-red from human glucocorticoid receptor by fluorescent polarization assay | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50303812 ((S)-1-(5-Chlorothiophen-2-yl)-1-((5aR,6S)-1-(4-flu...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 409 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to progesterone receptor | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303812 ((S)-1-(5-Chlorothiophen-2-yl)-1-((5aR,6S)-1-(4-flu...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of PMA-induced AP1 activity after 6 hrs by l... | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen Receptor (Homo sapiens (Human)) | BDBM50303812 ((S)-1-(5-Chlorothiophen-2-yl)-1-((5aR,6S)-1-(4-flu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to androgen receptor | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303812 ((S)-1-(5-Chlorothiophen-2-yl)-1-((5aR,6S)-1-(4-flu...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of IL-1-beta-induced NF-kappaB dependent E-s... | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50303812 ((S)-1-(5-Chlorothiophen-2-yl)-1-((5aR,6S)-1-(4-flu...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 253 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Transactivation of GAL-4 tagged glucocorticoid receptor ligand binding domain expressed in human HeLa cells assessed as NP1 activation by luciferase ... | J Med Chem 53: 1270-80 (2010) Article DOI: 10.1021/jm901551w BindingDB Entry DOI: 10.7270/Q2KS6SGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||