

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50304300 CHEMBL595573::trans-N-[1-(2-fluorophenyl)-3-pyrazolyl]-3-oxospiro[6-azaisobenzofuran-1(3H),1'-cyclohexane]-4'-carboxamide
SMILES: Fc1ccccc1-n1ccc(NC(=O)[C@H]2CC[C@@]3(CC2)OC(=O)c2ccncc32)n1
InChI Key: InChIKey=RMYZIRFUCOMQRH-CAJLXGCNSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50304300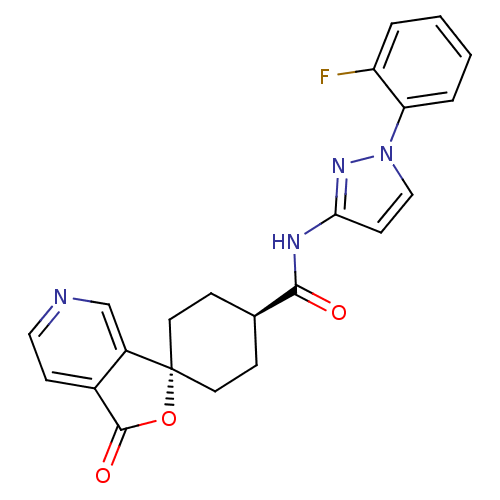 (CHEMBL595573 | trans-N-[1-(2-fluorophenyl)-3-pyraz...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in insect Sf9 membranes | Bioorg Med Chem Lett 22: 2738-43 (2012) Article DOI: 10.1016/j.bmcl.2012.02.098 BindingDB Entry DOI: 10.7270/Q2154J34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50304300 (CHEMBL595573 | trans-N-[1-(2-fluorophenyl)-3-pyraz...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonistic activity at human NPY Y5 receptor expressed in HEK293 cells assessed as inhibition of calcium level by FLIPR assay | Bioorg Med Chem Lett 20: 7120-3 (2010) Article DOI: 10.1016/j.bmcl.2010.09.064 BindingDB Entry DOI: 10.7270/Q2R49S2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50304300 (CHEMBL595573 | trans-N-[1-(2-fluorophenyl)-3-pyraz...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of rat Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50304300 (CHEMBL595573 | trans-N-[1-(2-fluorophenyl)-3-pyraz...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||