

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50304360 BENZENE-1,2,3,4-TETRAYL TETRAKIS[DIHYDROGEN (PHOSPHATE)]::CHEMBL595349::benzene-1,2,3,4-tetrayl tetrakis(hydrogen phosphate)
SMILES: OP(O)(=O)Oc1ccc(OP(O)(O)=O)c(OP(O)(O)=O)c1OP(O)(O)=O
InChI Key: InChIKey=UKRGHRHHBNLNDD-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50304360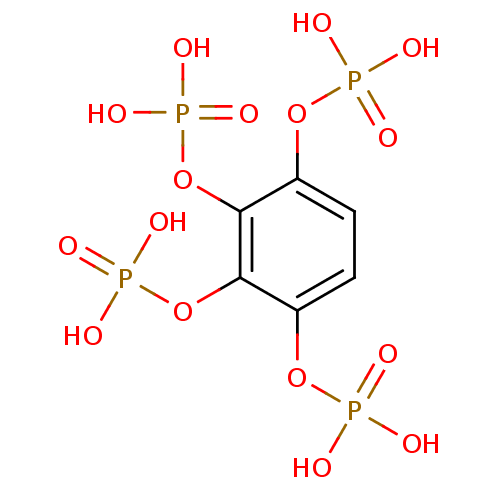 (BENZENE-1,2,3,4-TETRAYL TETRAKIS[DIHYDROGEN (PHOSP...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas Curated by ChEMBL | Assay Description Inhibition of pleckstrin homology domain of AKT by surface plasmon resonance spectroscopy | Bioorg Med Chem 17: 6983-92 (2009) Article DOI: 10.1016/j.bmc.2009.08.022 BindingDB Entry DOI: 10.7270/Q2513Z8T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Src homology-2 domain containing protein tyrosine phosphatase-2 (SHP2) (Homo sapiens (Human)) | BDBM50304360 (BENZENE-1,2,3,4-TETRAYL TETRAKIS[DIHYDROGEN (PHOSP...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Biological Sciences, UEA Curated by ChEMBL | Assay Description Displacement of 2FAMInsP5 from recombinant human N-terminal His-tagged SHIP2 (419 to 832 residues) expressed in Escherichia coli Rosetta2 (DE3) cells... | ACS Med Chem Lett 11: 309-315 (2020) Article DOI: 10.1021/acsmedchemlett.9b00368 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type I inositol-1,4,5-trisphosphate 5-phosphatase (Homo sapiens (Human)) | BDBM50304360 (BENZENE-1,2,3,4-TETRAYL TETRAKIS[DIHYDROGEN (PHOSP...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Biological Sciences, UEA Curated by ChEMBL | Assay Description Inhibition of recombinant human brain INPP5A expressed in Escherichia coli incubated for 10 mins by malachite green reagent based phosphate assay | ACS Med Chem Lett 11: 309-315 (2020) Article DOI: 10.1021/acsmedchemlett.9b00368 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||