

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50304489 CHEMBL592969::H-Tyr-c[D-Cys-Gly-Phe-Nle-Pro-D-Cys]-Trp-NH-[3',5'-(CF3)2Bzl]
SMILES: CCCC[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H](CSSC[C@@H](NC(=O)[C@@H]2CCCN2C1=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F)NC(=O)[C@@H](N)Cc1ccc(O)cc1
InChI Key: InChIKey=CYKFSGXEZOAASR-KMWMRXANSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurokinin 1 receptor (Homo sapiens (Human)) | BDBM50304489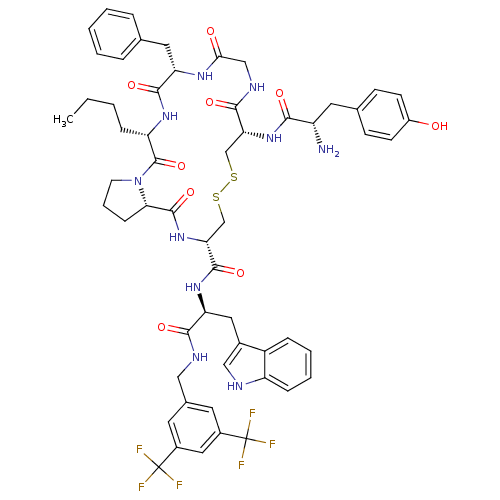 (CHEMBL592969 | H-Tyr-c[D-Cys-Gly-Phe-Nle-Pro-D-Cys...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membranes | Bioorg Med Chem 17: 7337-43 (2009) Article DOI: 10.1016/j.bmc.2009.08.035 BindingDB Entry DOI: 10.7270/Q2CJ8DK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurokinin 1 receptor (Rattus norvegicus (rat)) | BDBM50304489 (CHEMBL592969 | H-Tyr-c[D-Cys-Gly-Phe-Nle-Pro-D-Cys...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cell membranes | Bioorg Med Chem 17: 7337-43 (2009) Article DOI: 10.1016/j.bmc.2009.08.035 BindingDB Entry DOI: 10.7270/Q2CJ8DK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurokinin 1 receptor (Rattus norvegicus (rat)) | BDBM50304489 (CHEMBL592969 | H-Tyr-c[D-Cys-Gly-Phe-Nle-Pro-D-Cys...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from rat NK1 receptor expressed in CHO cell membranes | Bioorg Med Chem 17: 7337-43 (2009) Article DOI: 10.1016/j.bmc.2009.08.035 BindingDB Entry DOI: 10.7270/Q2CJ8DK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50304489 (CHEMBL592969 | H-Tyr-c[D-Cys-Gly-Phe-Nle-Pro-D-Cys...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in HN9.10 cells | Bioorg Med Chem 17: 7337-43 (2009) Article DOI: 10.1016/j.bmc.2009.08.035 BindingDB Entry DOI: 10.7270/Q2CJ8DK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50304489 (CHEMBL592969 | H-Tyr-c[D-Cys-Gly-Phe-Nle-Pro-D-Cys...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor expressed in HN9.10 cells | Bioorg Med Chem 17: 7337-43 (2009) Article DOI: 10.1016/j.bmc.2009.08.035 BindingDB Entry DOI: 10.7270/Q2CJ8DK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50304489 (CHEMBL592969 | H-Tyr-c[D-Cys-Gly-Phe-Nle-Pro-D-Cys...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at delta opioid receptor in mouse vas deferens assessed as inhibition of electrically-stimulated contraction | Bioorg Med Chem 17: 7337-43 (2009) Article DOI: 10.1016/j.bmc.2009.08.035 BindingDB Entry DOI: 10.7270/Q2CJ8DK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50304489 (CHEMBL592969 | H-Tyr-c[D-Cys-Gly-Phe-Nle-Pro-D-Cys...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in HN9.10 cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem 17: 7337-43 (2009) Article DOI: 10.1016/j.bmc.2009.08.035 BindingDB Entry DOI: 10.7270/Q2CJ8DK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurokinin 1 receptor (Homo sapiens (Human)) | BDBM50304489 (CHEMBL592969 | H-Tyr-c[D-Cys-Gly-Phe-Nle-Pro-D-Cys...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human NK1 receptor expressed in CHO cell membranes | Bioorg Med Chem 17: 7337-43 (2009) Article DOI: 10.1016/j.bmc.2009.08.035 BindingDB Entry DOI: 10.7270/Q2CJ8DK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50304489 (CHEMBL592969 | H-Tyr-c[D-Cys-Gly-Phe-Nle-Pro-D-Cys...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at rat mu opioid receptor expressed in HN9.10 cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem 17: 7337-43 (2009) Article DOI: 10.1016/j.bmc.2009.08.035 BindingDB Entry DOI: 10.7270/Q2CJ8DK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50304489 (CHEMBL592969 | H-Tyr-c[D-Cys-Gly-Phe-Nle-Pro-D-Cys...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Agonist activity at human delta opioid receptor expressed in HN9.10 cells assessed as stimulation of [35S]GTPgammaS binding | Bioorg Med Chem 17: 7337-43 (2009) Article DOI: 10.1016/j.bmc.2009.08.035 BindingDB Entry DOI: 10.7270/Q2CJ8DK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||