

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50304585 (20S)-1alpha,25-Dihydroxy-2-methylene-18,19-dinorvitamin D3::CHEMBL596532
SMILES: [#6]-[#6@@H](-[#6]-[#6]-[#6]C([#6])([#6])[#8])-[#6@H]-1-[#6]-[#6]-[#6@@H]2-[#6@@H]-1-[#6]-[#6]-[#6]\[#6]-2=[#6]/[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1
InChI Key: InChIKey=LTTDETGPYPVMCP-QOFIXJPPSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D receptor (Rattus norvegicus) | BDBM50304585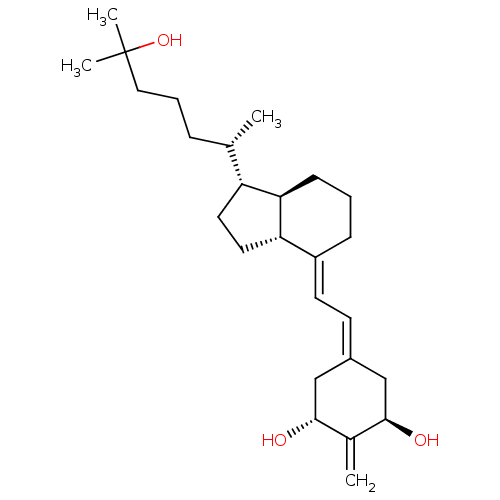 ((20S)-1alpha,25-Dihydroxy-2-methylene-18,19-dinorv...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of radiolabeled 1alpha,25-(OH)2D3 from rat recombinant full length VDR | Bioorg Med Chem 17: 7658-69 (2009) Article DOI: 10.1016/j.bmc.2009.09.047 BindingDB Entry DOI: 10.7270/Q26T0MQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D receptor (Rattus norvegicus) | BDBM50304585 ((20S)-1alpha,25-Dihydroxy-2-methylene-18,19-dinorv...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Activity at VDR in rat osteosarcoma cells assessed as induction of 24-hydroxylase reporter gene transcription by luciferase reporter gene assay | Bioorg Med Chem 17: 7658-69 (2009) Article DOI: 10.1016/j.bmc.2009.09.047 BindingDB Entry DOI: 10.7270/Q26T0MQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||