

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50304639 9-(5'-Cyclopropylmethylaminocarbonyl-4'-thio-beta-D-ribofuranosyl)-N6-(3-iodobenzyl)adenine::CHEMBL603424
SMILES: O[C@H]1[C@@H](O)[C@@H](S[C@@H]1C(=O)NCC1CC1)n1cnc2c(NCc3cccc(I)c3)ncnc12
InChI Key: InChIKey=WJGVCGYKHQZFKY-GRXQJBFDSA-N
Data: 8 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50304639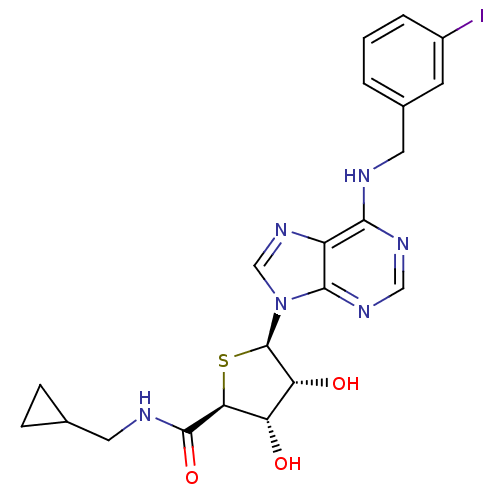 (9-(5'-Cyclopropylmethylaminocarbonyl-4'-thio-beta-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University Curated by ChEMBL | Assay Description Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARgamma LBD by TR-FRET assay | J Med Chem 60: 7459-7475 (2017) Article DOI: 10.1021/acs.jmedchem.7b00805 BindingDB Entry DOI: 10.7270/Q2XK8HQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50304639 (9-(5'-Cyclopropylmethylaminocarbonyl-4'-thio-beta-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor expressed in CHO cells | Bioorg Med Chem 17: 8003-11 (2009) Article DOI: 10.1016/j.bmc.2009.10.011 BindingDB Entry DOI: 10.7270/Q29K4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50304639 (9-(5'-Cyclopropylmethylaminocarbonyl-4'-thio-beta-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human A3 adenosine receptor expressed in CHO cell membranes after 60 mins by gamma counting method | J Med Chem 60: 7459-7475 (2017) Article DOI: 10.1021/acs.jmedchem.7b00805 BindingDB Entry DOI: 10.7270/Q2XK8HQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50304639 (9-(5'-Cyclopropylmethylaminocarbonyl-4'-thio-beta-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University Curated by ChEMBL | Assay Description Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay | J Med Chem 60: 7459-7475 (2017) Article DOI: 10.1021/acs.jmedchem.7b00805 BindingDB Entry DOI: 10.7270/Q2XK8HQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304639 (9-(5'-Cyclopropylmethylaminocarbonyl-4'-thio-beta-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 159 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]R-PIA from human adenosine A1 receptor expressed in CHO cells | Bioorg Med Chem 17: 8003-11 (2009) Article DOI: 10.1016/j.bmc.2009.10.011 BindingDB Entry DOI: 10.7270/Q29K4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50304639 (9-(5'-Cyclopropylmethylaminocarbonyl-4'-thio-beta-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 159 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University Curated by ChEMBL | Assay Description Displacement of [3H]CCPA from human A1 receptor expressed in CHO cell membranes after 60 mins by gamma counting method | J Med Chem 60: 7459-7475 (2017) Article DOI: 10.1021/acs.jmedchem.7b00805 BindingDB Entry DOI: 10.7270/Q2XK8HQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50304639 (9-(5'-Cyclopropylmethylaminocarbonyl-4'-thio-beta-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human A2A adenosine receptor expressed in HEK293 cell membranes after 60 mins by gamma counting method | J Med Chem 60: 7459-7475 (2017) Article DOI: 10.1021/acs.jmedchem.7b00805 BindingDB Entry DOI: 10.7270/Q2XK8HQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50304639 (9-(5'-Cyclopropylmethylaminocarbonyl-4'-thio-beta-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human adenosine A2A receptor expressed in CHO cells | Bioorg Med Chem 17: 8003-11 (2009) Article DOI: 10.1016/j.bmc.2009.10.011 BindingDB Entry DOI: 10.7270/Q29K4B9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||