 Found 70 hits for monomerid = 50305083
Found 70 hits for monomerid = 50305083 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083
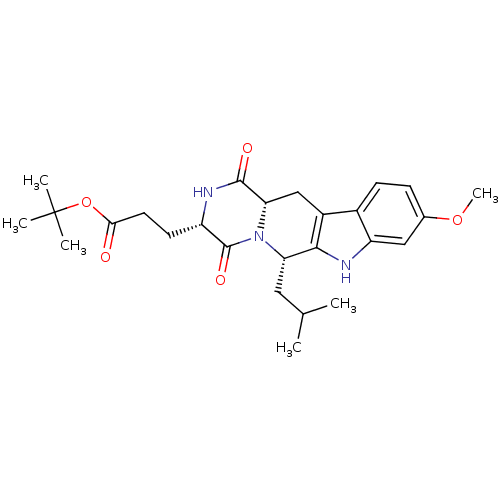
(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2-mediated mitoxantrone efflux in mitoxantrone-selected human H460 cells after 30 mins by flow cytometric analysis |
J Med Chem 56: 9849-60 (2013)
Article DOI: 10.1021/jm401649j
BindingDB Entry DOI: 10.7270/Q2RJ4NF5 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin)-mediated mitoxantrone efflux expressed in HEK293 cells after 30 mins by flow cytometric analysis |
J Med Chem 56: 9849-60 (2013)
Article DOI: 10.1021/jm401649j
BindingDB Entry DOI: 10.7270/Q2RJ4NF5 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
BMSSI UMR 5086 CNRS/Universit£ Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of human ABCG2 expressed in Sf9 insect cell membranes assessed as inhibition of quercetin-stimulated ATPase activity after 30 mins by colo... |
J Med Chem 56: 9849-60 (2013)
Article DOI: 10.1021/jm401649j
BindingDB Entry DOI: 10.7270/Q2RJ4NF5 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2 (unknown origin) expressed in MDCK2 cells co-expressing BCRP (unknown origin) assessed as effect on pheophorbide A accumulation p... |
J Med Chem 62: 4383-4400 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01821
BindingDB Entry DOI: 10.7270/Q2X06BDS |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of GFP-fused human ABCG2 expressed in MDCK2 cells assessed as Hoechst 33342 accumulation preincubated for 30 mins followed by Hoechst 3334... |
Eur J Med Chem 161: 506-525 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.026
BindingDB Entry DOI: 10.7270/Q23N26P9 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 221 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal GFP-tagged human ABCG2 expressed in MDCK2 cells preincubated for 30 mins before Hoechst 33342 addition by Hoechst 33342 accu... |
J Med Chem 59: 5449-61 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00330
BindingDB Entry DOI: 10.7270/Q2HH6PJ5 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 221 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of c-terminal GFP-tagged human ABCG2 expressed in MDCK2 cells preincubated for 30 mins before Hoechst 33342 addition by Hoechst 33342 accu... |
J Med Chem 59: 5449-61 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00330
BindingDB Entry DOI: 10.7270/Q2HH6PJ5 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human ABCG2 expressed in baculovirus infected Sf9 cell membrane assessed as inhibition of vanadate sensitive basal ATPase activity afte... |
J Med Chem 59: 6121-35 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00035
BindingDB Entry DOI: 10.7270/Q28K7DK6 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human ABCG2 expressed in baculovirus infected Sf9 cell membrane assessed as inhibition of vanadate sensitive quercetin-stimulated ATPas... |
J Med Chem 59: 6121-35 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00035
BindingDB Entry DOI: 10.7270/Q28K7DK6 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 221 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human ABCG2 expressed in MDCK2 cells assessed as reduction in Hoechst 33342 efflux pre-incubated for 30 mins before Hoechst 33342 addit... |
J Med Chem 59: 6121-35 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00035
BindingDB Entry DOI: 10.7270/Q28K7DK6 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ABCG2 in topotecan-cultured human MCF7 cells using Hoechst 33342 as substrate measured after 2 hrs by fluorescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112133
BindingDB Entry DOI: 10.7270/Q2MP56XK |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ABCC1 transfected in MDCK2-MRP1 cells using calcein-AM as substrate measured after 1 hr by fluorescence assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112133
BindingDB Entry DOI: 10.7270/Q2MP56XK |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activation of ABCB1 ATPase activity (unknown origin) expressed in baculovirus infected Sf9 insect cells using ATP as substrate measured after 1 hr by... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112133
BindingDB Entry DOI: 10.7270/Q2MP56XK |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 271 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of sulfasalazine-stimulated ABCB1 ATPase activity (unknown origin) expressed in baculovirus infected Sf9 insect cells using ATP as substra... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112133
BindingDB Entry DOI: 10.7270/Q2MP56XK |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ABCG2 (unknown origin) in HEK293 cells assessed as maximal inhibition of mitoxantrone efflux by fluorescence based flow cytometry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112503
BindingDB Entry DOI: 10.7270/Q29027FW |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ABCG2 expressed in MDCK2 cells co-expressing GFP preincubated for 30 mins followed by Hoechst 33342 addition and measured every 6... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00961
BindingDB Entry DOI: 10.7270/Q2ZS317X |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 274 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ABCG2 expressed in MDCK2 cells co-expressing GFP preincubated for 15 mins followed by pheophorbideA addition and measured after 2... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00961
BindingDB Entry DOI: 10.7270/Q2ZS317X |
More data for this
Ligand-Target Pair | |
Multidrug resistance-associated protein 1
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of ABCC1 mediated etoposide resistant in human 2008 cells |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113403
BindingDB Entry DOI: 10.7270/Q2Q2442W |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Reversal of P-gp-mediated multidrug resistance in human K562/A02 cells assessed as potentiation of adriamycin-induced cytotoxicity at 5 uM by measuri... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00246
BindingDB Entry DOI: 10.7270/Q29Z98N9 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50305083

(3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,...)Show SMILES COc1ccc2c3C[C@@H]4N([C@@H](CC(C)C)c3[nH]c2c1)C(=O)[C@H](CCC(=O)OC(C)(C)C)NC4=O |r| Show InChI InChI=1S/C26H35N3O5/c1-14(2)11-20-23-17(16-8-7-15(33-6)12-19(16)27-23)13-21-24(31)28-18(25(32)29(20)21)9-10-22(30)34-26(3,4)5/h7-8,12,14,18,20-21,27H,9-11,13H2,1-6H3,(H,28,31)/t18-,20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114346
BindingDB Entry DOI: 10.7270/Q2PZ5DT3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data