

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: OC(CNC(=O)c1ccc(nn1)N1CCC2(CC1)CC(=O)c1ccccc1O2)c1ccccc1
InChI Key: InChIKey=OPPYXCWIDMCXMM-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306110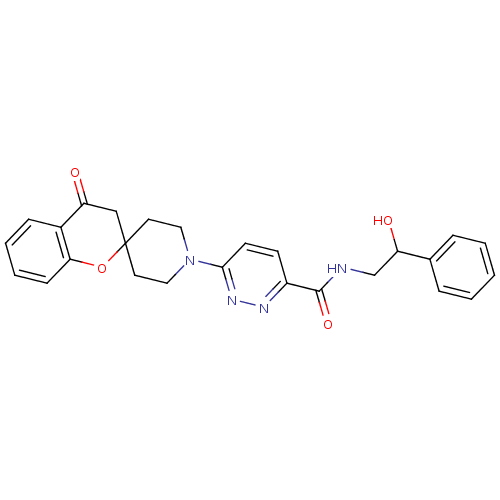 (CHEMBL607314 | N-(2-hydroxy-2-phenylethyl)-6-(4-ox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate after 60 mins in presence of S9 microsomal fraction | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50306110 (CHEMBL607314 | N-(2-hydroxy-2-phenylethyl)-6-(4-ox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomal liver S9 microsomal fraction assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50306110 (CHEMBL607314 | N-(2-hydroxy-2-phenylethyl)-6-(4-ox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse liver microsome SCD1 | Eur J Med Chem 46: 1892-6 (2011) Article DOI: 10.1016/j.ejmech.2011.02.002 BindingDB Entry DOI: 10.7270/Q25T3KTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306110 (CHEMBL607314 | N-(2-hydroxy-2-phenylethyl)-6-(4-ox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human liver microsome SCD1 | Eur J Med Chem 46: 1892-6 (2011) Article DOI: 10.1016/j.ejmech.2011.02.002 BindingDB Entry DOI: 10.7270/Q25T3KTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306110 (CHEMBL607314 | N-(2-hydroxy-2-phenylethyl)-6-(4-ox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||