

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50307836 CHEMBL608559::Sodium 1-Amino-4-(2-anthracenylamino)-9,10-dioxo-9,10-dihydroanthracene-2-sulfonate
SMILES: Nc1c2C(=O)c3ccccc3C(=O)c2c(Nc2ccc3cc4ccccc4cc3c2)cc1S([O-])(=O)=O
InChI Key: InChIKey=JXFRJOYKTCEJDG-UHFFFAOYSA-M
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ecto-5'-nucleotidase (e5'NT) (Rattus norvegicus (Rat)) | BDBM50307836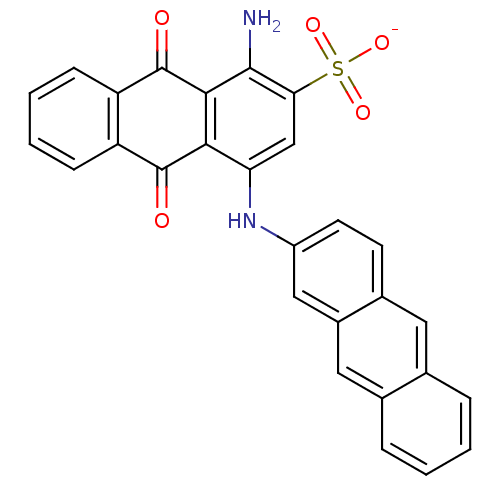 (CHEMBL608559 | Sodium 1-Amino-4-(2-anthracenylamin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of rat ecto-5'-nucleotidase expressed in Sf9 cells by capillary electrophoresis method | J Med Chem 53: 2076-86 (2010) Article DOI: 10.1021/jm901851t BindingDB Entry DOI: 10.7270/Q2DZ097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleoside triphosphate diphosphohydrolase 1 (Homo sapiens (Human)) | BDBM50307836 (CHEMBL608559 | Sodium 1-Amino-4-(2-anthracenylamin...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human NTPdase1 by capillary electrophoresis method | J Med Chem 53: 2076-86 (2010) Article DOI: 10.1021/jm901851t BindingDB Entry DOI: 10.7270/Q2DZ097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleoside triphosphate diphosphohydrolase 3 (Homo sapiens (Human)) | BDBM50307836 (CHEMBL608559 | Sodium 1-Amino-4-(2-anthracenylamin...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human NTPdase3 by capillary electrophoresis method | J Med Chem 53: 2076-86 (2010) Article DOI: 10.1021/jm901851t BindingDB Entry DOI: 10.7270/Q2DZ097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleoside triphosphate diphosphohydrolase 2 (Homo sapiens (Human)) | BDBM50307836 (CHEMBL608559 | Sodium 1-Amino-4-(2-anthracenylamin...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human NTPdase2 by capillary electrophoresis method | J Med Chem 53: 2076-86 (2010) Article DOI: 10.1021/jm901851t BindingDB Entry DOI: 10.7270/Q2DZ097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyrimidinergic receptor P2Y4 (Homo sapiens (Human)) | BDBM50307836 (CHEMBL608559 | Sodium 1-Amino-4-(2-anthracenylamin...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at human P2Y4 receptor expressed in astrocytoma cells assessed as inhibition of intracellular calcium mobilization | J Med Chem 53: 2076-86 (2010) Article DOI: 10.1021/jm901851t BindingDB Entry DOI: 10.7270/Q2DZ097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purinergic receptor P2Y2 (Homo sapiens (Human)) | BDBM50307836 (CHEMBL608559 | Sodium 1-Amino-4-(2-anthracenylamin...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at human P2Y2 receptor expressed in astrocytoma cells assessed as inhibition of intracellular calcium mobilization | J Med Chem 53: 2076-86 (2010) Article DOI: 10.1021/jm901851t BindingDB Entry DOI: 10.7270/Q2DZ097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyrimidinergic receptor P2Y6 (Rattus norvegicus) | BDBM50307836 (CHEMBL608559 | Sodium 1-Amino-4-(2-anthracenylamin...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at rat P2Y6 receptor expressed in astrocytoma cells assessed as inhibition of intracellular calcium mobilization | J Med Chem 53: 2076-86 (2010) Article DOI: 10.1021/jm901851t BindingDB Entry DOI: 10.7270/Q2DZ097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||