

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50307942 4-Amino-N-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide::CHEMBL598194::US8796293, 69
SMILES: NC1(CCN(CC1)c1ncnc2[nH]ccc12)C(=O)NCc1ccc(Cl)cc1
InChI Key: InChIKey=XQPFDQVEBGYLHB-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50307942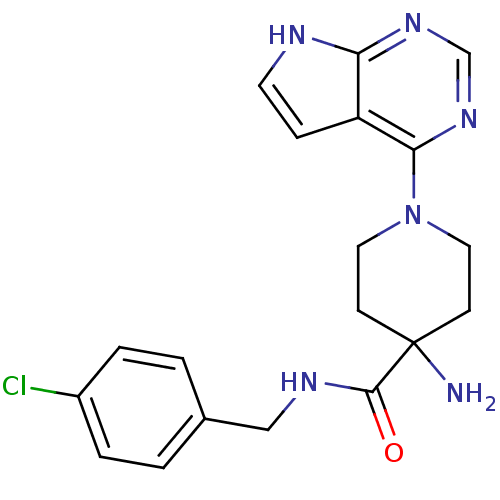 (4-Amino-N-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyr...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 143 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astex Therapeutics Limited; The Institute of Cancer Research: Royal Cancer Hospital; Cancer Research Technology Limited US Patent | Assay Description In a final reaction volume of 25 ul, ROCK-II (h) (5-10 mU) is incubated with 50 mM Tris pH 7.5, 0.1 mM EGTA, 30 uM KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK (... | US Patent US8796293 (2014) BindingDB Entry DOI: 10.7270/Q21C1VJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50307942 (4-Amino-N-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human microsomal preparation | J Med Chem 53: 2239-49 (2010) Article DOI: 10.1021/jm901788j BindingDB Entry DOI: 10.7270/Q23B608V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50307942 (4-Amino-N-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyr...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human microsomal preparation | J Med Chem 53: 2239-49 (2010) Article DOI: 10.1021/jm901788j BindingDB Entry DOI: 10.7270/Q23B608V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50307942 (4-Amino-N-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human microsomal preparation | J Med Chem 53: 2239-49 (2010) Article DOI: 10.1021/jm901788j BindingDB Entry DOI: 10.7270/Q23B608V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50307942 (4-Amino-N-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human microsomal preparation | J Med Chem 53: 2239-49 (2010) Article DOI: 10.1021/jm901788j BindingDB Entry DOI: 10.7270/Q23B608V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50307942 (4-Amino-N-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by ionworks assay | J Med Chem 56: 2059-73 (2013) Article DOI: 10.1021/jm301762v BindingDB Entry DOI: 10.7270/Q2QR4ZFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50307942 (4-Amino-N-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of PKBbeta by radiometric filter binding assay | J Med Chem 53: 2239-49 (2010) Article DOI: 10.1021/jm901788j BindingDB Entry DOI: 10.7270/Q23B608V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50307942 (4-Amino-N-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Akt2 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass... | J Med Chem 56: 2059-73 (2013) Article DOI: 10.1021/jm301762v BindingDB Entry DOI: 10.7270/Q2QR4ZFN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50307942 (4-Amino-N-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyr...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Akt1 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass... | J Med Chem 56: 2059-73 (2013) Article DOI: 10.1021/jm301762v BindingDB Entry DOI: 10.7270/Q2QR4ZFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50307942 (4-Amino-N-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyr...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant ROCK2 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift as... | J Med Chem 56: 2059-73 (2013) Article DOI: 10.1021/jm301762v BindingDB Entry DOI: 10.7270/Q2QR4ZFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50307942 (4-Amino-N-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant Akt3 (unknown origin) using 5-FAM-labeled peptide as substrate after 1 hr by caliper off-chip incubation mobility shift ass... | J Med Chem 56: 2059-73 (2013) Article DOI: 10.1021/jm301762v BindingDB Entry DOI: 10.7270/Q2QR4ZFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50307942 (4-Amino-N-(4-chlorobenzyl)-1-(7H-pyrrolo[2,3-d]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human microsomal preparation | J Med Chem 53: 2239-49 (2010) Article DOI: 10.1021/jm901788j BindingDB Entry DOI: 10.7270/Q23B608V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||