

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50309494 (1R,2S,3R,5S)-3-(2-(2-(1-ethyl-1H-imidazol-4-yl)ethylamino)-6-(2-ethylbutylamino)-9H-purin-9-yl)-5-(4-(hydroxymethyl)-1H-pyrazol-1-yl)cyclopentane-1,2-diol::CHEMBL604591
SMILES: CCC(CC)CNc1nc(NCCc2cn(CC)cn2)nc2n(cnc12)[C@@H]1C[C@@H]([C@@H](O)[C@H]1O)n1cc(CO)cn1
InChI Key: InChIKey=NCSZDLWKVPXZCB-AWAHEQQVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309494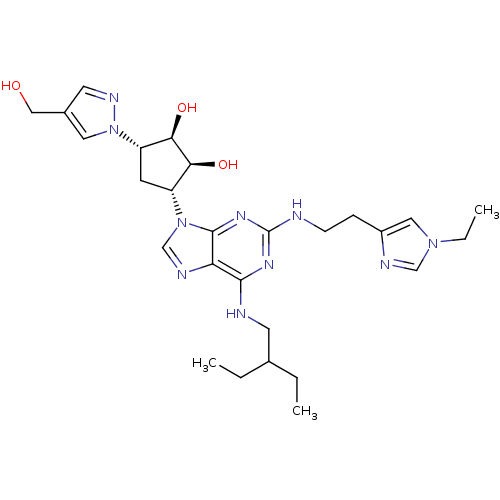 ((1R,2S,3R,5S)-3-(2-(2-(1-ethyl-1H-imidazol-4-yl)et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50309494 ((1R,2S,3R,5S)-3-(2-(2-(1-ethyl-1H-imidazol-4-yl)et...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 789 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A3 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50309494 ((1R,2S,3R,5S)-3-(2-(2-(1-ethyl-1H-imidazol-4-yl)et...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A2B receptor expressed in CHO cells assessed as effect on cAMP production by luciferase reporter gene assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50309494 ((1R,2S,3R,5S)-3-(2-(2-(1-ethyl-1H-imidazol-4-yl)et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at adenosine A2A receptor in human neutrophils assessed as inhibition of fMLP-induced reactive oxygen species release by chemilumine... | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50309494 ((1R,2S,3R,5S)-3-(2-(2-(1-ethyl-1H-imidazol-4-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells by GTPPgammaS binding assay | Bioorg Med Chem Lett 20: 1219-24 (2010) Article DOI: 10.1016/j.bmcl.2009.11.131 BindingDB Entry DOI: 10.7270/Q2X92BDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||