

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CN(C)CCCNC(=O)c1ccc2OCC(Cc2c1)c1nc2ccc(cc2s1)-c1cn[nH]c1
InChI Key: InChIKey=MUVYYVZAJFICKR-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50311728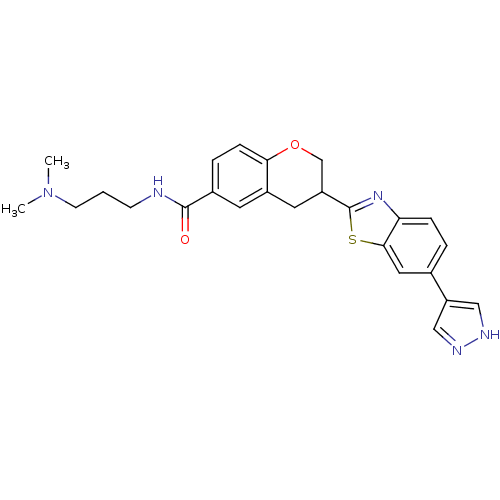 (3-(6-(1H-pyrazol-4-yl)benzo[d]thiazol-2-yl)-N-(3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute and Department of Molecular Therapeutics Curated by ChEMBL | Assay Description Inhibition of ROCK2 | Bioorg Med Chem Lett 19: 6686-90 (2009) Article DOI: 10.1016/j.bmcl.2009.09.115 BindingDB Entry DOI: 10.7270/Q2VX0GN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50311728 (3-(6-(1H-pyrazol-4-yl)benzo[d]thiazol-2-yl)-N-(3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute and Department of Molecular Therapeutics Curated by ChEMBL | Assay Description Inhibition of ROCK2 assessed as myosin light chain phosphorylation by cell-based assay | Bioorg Med Chem Lett 19: 6686-90 (2009) Article DOI: 10.1016/j.bmcl.2009.09.115 BindingDB Entry DOI: 10.7270/Q2VX0GN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50311728 (3-(6-(1H-pyrazol-4-yl)benzo[d]thiazol-2-yl)-N-(3-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute and Department of Molecular Therapeutics Curated by ChEMBL | Assay Description Inhibition of JNK3 | Bioorg Med Chem Lett 19: 6686-90 (2009) Article DOI: 10.1016/j.bmcl.2009.09.115 BindingDB Entry DOI: 10.7270/Q2VX0GN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase MRCK alpha (Homo sapiens (Human)) | BDBM50311728 (3-(6-(1H-pyrazol-4-yl)benzo[d]thiazol-2-yl)-N-(3-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute and Department of Molecular Therapeutics Curated by ChEMBL | Assay Description Inhibition of MRCKalpha | Bioorg Med Chem Lett 19: 6686-90 (2009) Article DOI: 10.1016/j.bmcl.2009.09.115 BindingDB Entry DOI: 10.7270/Q2VX0GN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50311728 (3-(6-(1H-pyrazol-4-yl)benzo[d]thiazol-2-yl)-N-(3-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Translational Research Institute and Department of Molecular Therapeutics Curated by ChEMBL | Assay Description Inhibition of ROCK1 | Bioorg Med Chem Lett 19: 6686-90 (2009) Article DOI: 10.1016/j.bmcl.2009.09.115 BindingDB Entry DOI: 10.7270/Q2VX0GN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||