

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50313224 5-(4-tert-butylphenyl)-1-(4-isopropoxyphenyl)-1H-indole-2-carboxylic acid::CHEMBL1076694
SMILES: CC(C)Oc1ccc(cc1)-n1c(cc2cc(ccc12)-c1ccc(cc1)C(C)(C)C)C(O)=O
InChI Key: InChIKey=GUMCVDVWBMLUSQ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313224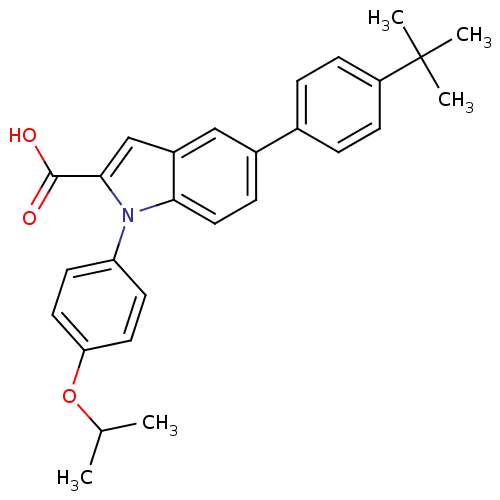 (5-(4-tert-butylphenyl)-1-(4-isopropoxyphenyl)-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Biotechnology Center San Diego Curated by ChEMBL | Assay Description Inhibition of recombinant human mPGES-1 expressed in human 293E cell microsomes using PGH2 as substrate assessed as PGE2 production after 2.5 mins by... | J Med Chem 58: 4727-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00330 BindingDB Entry DOI: 10.7270/Q23J3FQ0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313224 (5-(4-tert-butylphenyl)-1-(4-isopropoxyphenyl)-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313224 (5-(4-tert-butylphenyl)-1-(4-isopropoxyphenyl)-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Biotechnology Center San Diego Curated by ChEMBL | Assay Description Inhibition of purified mPGES-1 (1 to 152) (unknown origin) extracted from detergent-solubilized baculovirus-infected insect Sf9 cell membranes using ... | J Med Chem 58: 4727-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00330 BindingDB Entry DOI: 10.7270/Q23J3FQ0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||