

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50314627 (E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)piperidin-1-ylmethyl]-phenyl}acrylamide::CHEMBL1089343
SMILES: Cc1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2)C1
InChI Key: InChIKey=SDBLMAGBFSJKHB-OUKQBFOZSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50314627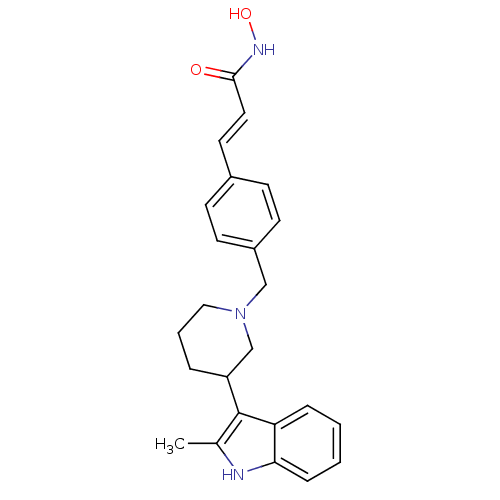 ((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells by automated patch clamp electrophisiology assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cereblon/Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314627 ((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of GST-tagged HDAC1 (unknown origin) expressed in insect cells using poly (Glu, Tyr) 4:1 as substrate measured after 15 mins in presence o... | Eur J Med Chem 158: 620-706 (2018) Article DOI: 10.1016/j.ejmech.2018.08.073 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50314627 ((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay | J Med Chem 53: 2952-63 (2010) Article DOI: 10.1021/jm100007m BindingDB Entry DOI: 10.7270/Q20V8CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||