

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50314760 2-(methylthio)-3-(phenylsulfonyl)-6,7,8,9-tetrahydropyrazolo[1,5-a]quinazoline::CHEMBL1091207::US8629154, 1.2(1)
SMILES: CSc1nn2c3CCCCc3cnc2c1S(=O)(=O)c1ccccc1
InChI Key: InChIKey=GALCAARNDAQXPY-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314760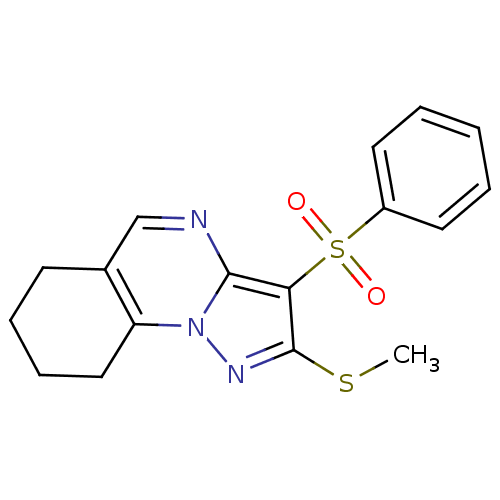 (2-(methylthio)-3-(phenylsulfonyl)-6,7,8,9-tetrahyd...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.549 | -12.8 | 1.18 | n/a | n/a | n/a | n/a | 7.4 | 30 |
TBA US Patent | Assay Description Determination of tested compounds binding with 5-HT6 receptors was carried out according to the method described in [Monsma F J Jr, Shen Y, Ward R P,... | US Patent US8629154 (2014) BindingDB Entry DOI: 10.7270/Q20R9N2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314760 (2-(methylthio)-3-(phenylsulfonyl)-6,7,8,9-tetrahyd...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.549 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5HT6 receptor in humanHeLa cells after 120 mins | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50314760 (2-(methylthio)-3-(phenylsulfonyl)-6,7,8,9-tetrahyd...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 411 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT2B receptor in HEK293 cells assessed as inhibition of alphaME-5-HT-induced cAMP accumulation pretreated for 15 secs b... | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314760 (2-(methylthio)-3-(phenylsulfonyl)-6,7,8,9-tetrahyd...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor in HEK293 cells assessed as inhibition of serotonin-induced cAMP accumulation pretreated for 15 mins befor... | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314760 (2-(methylthio)-3-(phenylsulfonyl)-6,7,8,9-tetrahyd...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of general formulas 1 and 2 were tested for their ability to prevent 5-HT6 receptors activation by serotonin. HEK 293 cells (cells of human... | US Patent US8629154 (2014) BindingDB Entry DOI: 10.7270/Q20R9N2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||