

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50314763 9-methyl-2-(methylthio)-3-(phenylsulfonyl)-5,6,7,8-tetrahydropyrazolo[5,1-b]quinazoline::CHEMBL1092241::US8629154, 2(2)
SMILES: CSc1nn2c(C)c3CCCCc3nc2c1S(=O)(=O)c1ccccc1
InChI Key: InChIKey=WKNMEUSGOJIXHX-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314763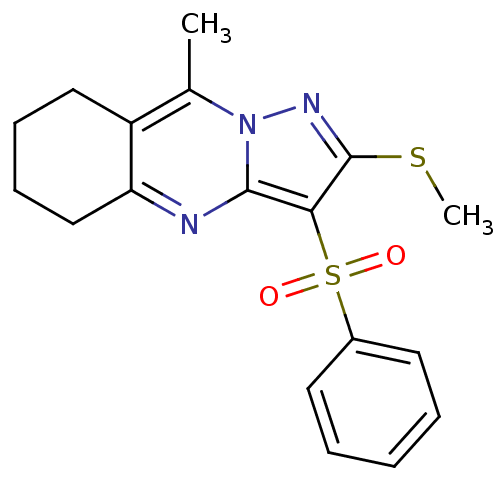 (9-methyl-2-(methylthio)-3-(phenylsulfonyl)-5,6,7,8...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 0.680 | -12.7 | 1.46 | n/a | n/a | n/a | n/a | 7.4 | 30 |
TBA US Patent | Assay Description Determination of tested compounds binding with 5-HT6 receptors was carried out according to the method described in [Monsma F J Jr, Shen Y, Ward R P,... | US Patent US8629154 (2014) BindingDB Entry DOI: 10.7270/Q20R9N2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314763 (9-methyl-2-(methylthio)-3-(phenylsulfonyl)-5,6,7,8...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5HT6 receptor in humanHeLa cells after 120 mins | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314763 (9-methyl-2-(methylthio)-3-(phenylsulfonyl)-5,6,7,8...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute (CDRI) Curated by ChEMBL | Assay Description Antagonist activity at 5-HT6 receptor (unknown origin) | Bioorg Med Chem 21: 4614-27 (2013) Article DOI: 10.1016/j.bmc.2013.05.040 BindingDB Entry DOI: 10.7270/Q2DJ5JK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314763 (9-methyl-2-(methylthio)-3-(phenylsulfonyl)-5,6,7,8...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT6 receptor in HEK293 cells assessed as inhibition of serotonin-induced cAMP accumulation pretreated for 15 mins befor... | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50314763 (9-methyl-2-(methylthio)-3-(phenylsulfonyl)-5,6,7,8...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Diversity Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human 5HT2B receptor in HEK293 cells assessed as inhibition of alphaME-5-HT-induced cAMP accumulation pretreated for 15 secs b... | Bioorg Med Chem Lett 20: 2133-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.046 BindingDB Entry DOI: 10.7270/Q2V40VBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50314763 (9-methyl-2-(methylthio)-3-(phenylsulfonyl)-5,6,7,8...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description Compounds of general formulas 1 and 2 were tested for their ability to prevent 5-HT6 receptors activation by serotonin. HEK 293 cells (cells of human... | US Patent US8629154 (2014) BindingDB Entry DOI: 10.7270/Q20R9N2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||