

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50315287 (R)-N1-((1H-benzo[d]imidazol-2-yl)methyl)-N1-(5,6,7,8-tetrahydroquinolin-8-yl)butane-1,4-diamine::CHEMBL1093149
SMILES: NCCCCN(Cc1nc2ccccc2[nH]1)[C@@H]1CCCc2cccnc12
InChI Key: InChIKey=WVLHHLRVNDMIAR-LJQANCHMSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 4 (CXCR4) (Homo sapiens (Human)) | BDBM50315287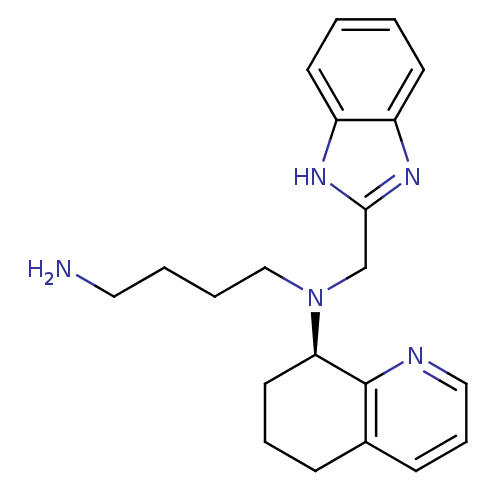 ((R)-N1-((1H-benzo[d]imidazol-2-yl)methyl)-N1-(5,6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 in human CEM-CCRF cells by liquid scintillation counting | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (CXCR4) (Homo sapiens (Human)) | BDBM50315287 ((R)-N1-((1H-benzo[d]imidazol-2-yl)methyl)-N1-(5,6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to human CXCR4 expressed in HEK293 cells | Bioorg Med Chem Lett 20: 3026-30 (2010) Article DOI: 10.1016/j.bmcl.2010.03.118 BindingDB Entry DOI: 10.7270/Q2J67H2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (CXCR4) (Homo sapiens (Human)) | BDBM50315287 ((R)-N1-((1H-benzo[d]imidazol-2-yl)methyl)-N1-(5,6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of [125I]SDF-1alpha from CXCR4 in human CEM-CCRF cells by liquid scintillation counting | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (CXCR4) (Homo sapiens (Human)) | BDBM50315287 ((R)-N1-((1H-benzo[d]imidazol-2-yl)methyl)-N1-(5,6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CEM-CCRF cells assessed as inhibition of SDF-1-induced calcium flux | J Med Chem 53: 3376-88 (2010) Article DOI: 10.1021/jm100073m BindingDB Entry DOI: 10.7270/Q2GQ6XW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||