

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50315857 (2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(4-methoxyphenylamino)-9H-purin-9-yl)tetrahydrofuran-3,4-diol::CHEMBL1093910
SMILES: COc1ccc(Nc2ncnc3n(cnc23)[C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)cc1
InChI Key: InChIKey=DHSVZMSVSTXYCS-LSCFUAHRSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50315857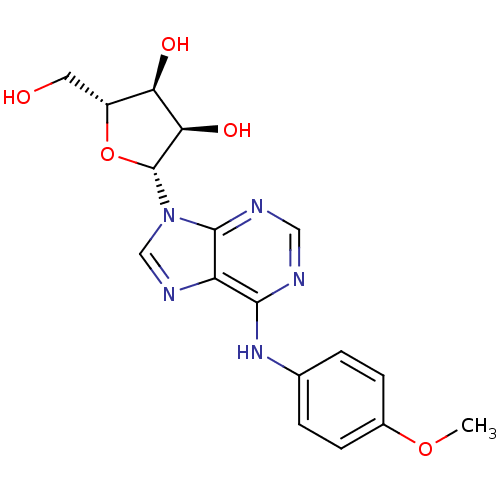 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(4-methoxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of binding of 1 nM N6-[3H]cyclohexyladenosine to adenosine A1 receptor on rat cerebral cortical membranes. | J Med Chem 28: 1341-6 (1985) BindingDB Entry DOI: 10.7270/Q2BP03CS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50315857 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(4-methoxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCX from human adenosine A1 receptor expressed in CHO cells at high affinity site by scintillation counting | J Med Chem 53: 3028-37 (2010) Article DOI: 10.1021/jm901252a BindingDB Entry DOI: 10.7270/Q20K28RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50315857 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(4-methoxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCX from human adenosine A1 receptor expressed in CHO cells by scintillation counting in presence of 10 uM allosteric modulator ... | J Med Chem 53: 3028-37 (2010) Article DOI: 10.1021/jm901252a BindingDB Entry DOI: 10.7270/Q20K28RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50315857 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(4-methoxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCX from human adenosine A1 receptor expressed in CHO cells by scintillation counting | J Med Chem 53: 3028-37 (2010) Article DOI: 10.1021/jm901252a BindingDB Entry DOI: 10.7270/Q20K28RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50315857 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(4-methoxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCX from human adenosine A1 receptor expressed in CHO cells at low affinity site by scintillation counting | J Med Chem 53: 3028-37 (2010) Article DOI: 10.1021/jm901252a BindingDB Entry DOI: 10.7270/Q20K28RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADORA1 (Chick) | BDBM50315857 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(4-methoxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [125I]ABA from adenosine A1 receptor of chick cerebellar membrane | J Med Chem 30: 954-6 (1987) BindingDB Entry DOI: 10.7270/Q2PN967D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50315857 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(4-methoxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Evaluated for binding affinity against Adenosine A1 receptor | J Med Chem 35: 629-35 (1992) Checked by Author BindingDB Entry DOI: 10.7270/Q23R0V5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine A2 receptor (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50315857 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(4-methoxyphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-NECA from adenosine A2 receptor of rat striatal membrane | J Med Chem 30: 954-6 (1987) BindingDB Entry DOI: 10.7270/Q2PN967D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50315857 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(4-methoxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells assessed as stimulation of ERK1/2 phosphorylation | J Med Chem 53: 3028-37 (2010) Article DOI: 10.1021/jm901252a BindingDB Entry DOI: 10.7270/Q20K28RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50315857 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(4-methoxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting in presence of 10... | J Med Chem 53: 3028-37 (2010) Article DOI: 10.1021/jm901252a BindingDB Entry DOI: 10.7270/Q20K28RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50315857 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(4-methoxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 142 | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting | J Med Chem 53: 3028-37 (2010) Article DOI: 10.1021/jm901252a BindingDB Entry DOI: 10.7270/Q20K28RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50315857 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(4-methoxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Evaluated for binding affinity against Adenosine A1 receptor | J Med Chem 35: 629-35 (1992) Checked by Author BindingDB Entry DOI: 10.7270/Q23R0V5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50315857 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-(4-methoxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells assessed as stimulation of ERK1/2 phosphorylation in presence of 10 uM alloste... | J Med Chem 53: 3028-37 (2010) Article DOI: 10.1021/jm901252a BindingDB Entry DOI: 10.7270/Q20K28RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||