

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50316180 (1S,2R,3S,4S)-1-{(1S)-2-[(2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)tetrahydrothiophenium-1-yl]-1-hydroxyethyl}-2,3,4,5-tetrahydroxypentyl sulfate::(2S,3S,4R,5S,6S)-1-((2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)tetrahydro-1H-thiophenium-1-yl)-2,4,5,6,7-pentahydroxyheptan-3-yl sulfate::CHEMBL1093524
SMILES: OC[C@H](O)[C@H](O)[C@@H](O)[C@H](OS([O-])(=O)=O)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO
InChI Key: InChIKey=OMKXVFDVAGCPBS-RAPQXRGMSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maltase-glucoamylase (Homo sapiens (Human)) | BDBM50316180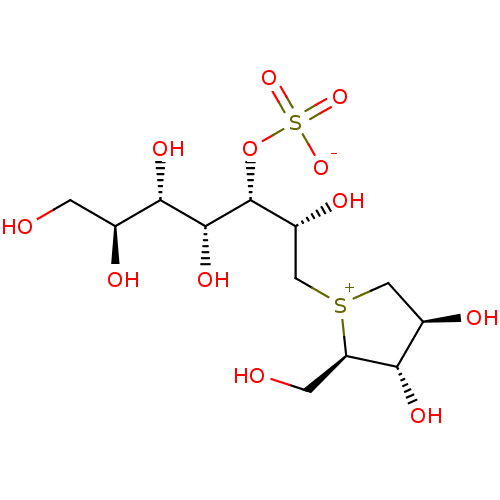 ((1S,2R,3S,4S)-1-{(1S)-2-[(2R,3S,4S)-3,4-dihydroxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University Curated by ChEMBL | Assay Description Inhibition of recombinant human maltase glucoamylase N-terminal catalytic domain | Bioorg Med Chem 18: 2829-35 (2010) Article DOI: 10.1016/j.bmc.2010.03.027 BindingDB Entry DOI: 10.7270/Q22B8Z5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||