

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: C(COCC[n+]1ccc2c(c1)[nH]c1ccccc21)OCCOCC[n+]1ccc2c(c1)[nH]c1ccccc21
InChI Key: InChIKey=BZSDSYSGDUHDMQ-UHFFFAOYSA-P
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, NMDA 1/2A (Homo sapiens (Human)) | BDBM50317191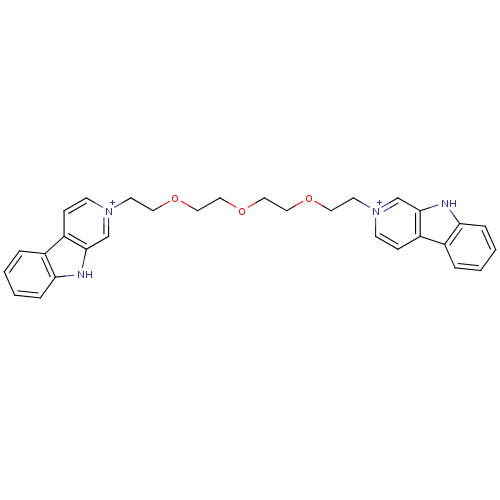 (2-[2-(2-{2-[2-(beta-Carboline-2-ium-2-yl)ethoxy]et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of dexamethasone-induced human NR1-1a/NR2A receptor-mediated excitotoxicity in (S)-glutamate/glycine-stimulated mouse L12-G10 cells assess... | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50317191 (2-[2-(2-{2-[2-(beta-Carboline-2-ium-2-yl)ethoxy]et...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of electric eel AChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50317191 (2-[2-(2-{2-[2-(beta-Carboline-2-ium-2-yl)ethoxy]et...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich-Schiller-Universitat Jena Curated by ChEMBL | Assay Description Inhibition of equine serum BChE by modified Ellman's method | J Med Chem 53: 3611-7 (2010) Article DOI: 10.1021/jm1000024 BindingDB Entry DOI: 10.7270/Q2SJ1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||