

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50317625 4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-5'-yloxy)-hexyl]phenoxy}butyric Acid::CHEMBL1099323
SMILES: OC(=O)CCCOc1cccc(CCCCCCOc2cc(cc(c2)-c2ccccc2)-c2ccccc2)c1CCC(O)=O
InChI Key: InChIKey=SMNCEXHPBATBIT-UHFFFAOYSA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene 2 (Homo sapiens (Human)) | BDBM50317625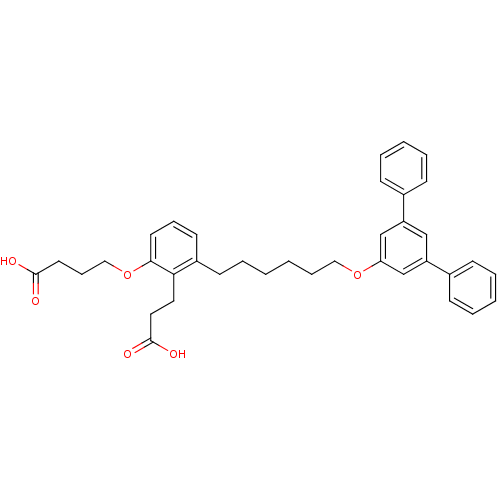 (4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 628 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at FLAG-tagged human BLT2 receptor expressed in HEK293 cells assessed as inhibition of LTB4-stimulated calcium mobilization prein... | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50317625 (4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of CYP2C9 by LC/MS method | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50317625 (4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHO cells assessed as reduction of current amplitude | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50317625 (4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of CYP2C9 fluorescence method | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor (Homo sapiens (Human)) | BDBM50317625 (4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at FLAG-tagged human BLT1 receptor expressed in HEK293 cells assessed as inhibition of LTB4-stimulated calcium mobilization prein... | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50317625 (4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of CYP2D6 flourescence method | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50317625 (4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of CYP2C19 flourescence method | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50317625 (4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of CYP3A4 fluorescence method | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor (Homo sapiens (Human)) | BDBM50317625 (4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50317625 (4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Inhibition of CYP1A2 by fluorescence method | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||