

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50318596 3-((4-(6-Bromo-2-(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo-[4,5-b]pyridin-7-yl)piperazin-1-yl)methyl)-5-methylisoxazole::CHEMBL1086579
SMILES: CN1CCN(CC1)c1nc2ncc(Br)c(N3CCN(Cc4cc(C)on4)CC3)c2[nH]1
InChI Key: InChIKey=FZTUPODAEPTYNG-UHFFFAOYSA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase B (Homo sapiens (Human)) | BDBM50318596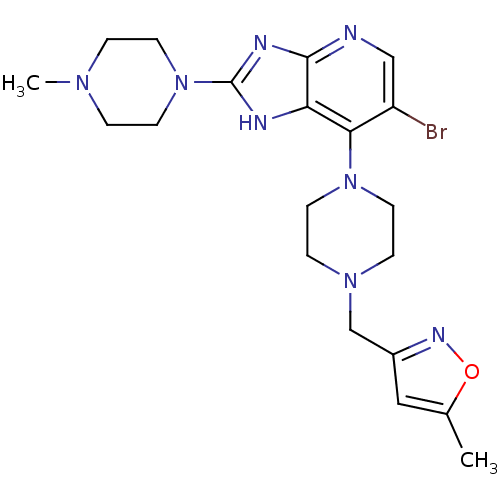 (3-((4-(6-Bromo-2-(4-(4-methylpiperazin-1-yl)phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant Aurora B expressed in baculovirus system | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50318596 (3-((4-(6-Bromo-2-(4-(4-methylpiperazin-1-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of CYP2A6 from human liver microsome by LC-MS/MS analysis | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase C (Homo sapiens (Human)) | BDBM50318596 (3-((4-(6-Bromo-2-(4-(4-methylpiperazin-1-yl)phenyl...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant Aurora C expressed in baculovirus system | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50318596 (3-((4-(6-Bromo-2-(4-(4-methylpiperazin-1-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of CYP1A2 from human liver microsome by LC-MS/MS analysis | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50318596 (3-((4-(6-Bromo-2-(4-(4-methylpiperazin-1-yl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant Aurora A expressed in baculovirus system | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50318596 (3-((4-(6-Bromo-2-(4-(4-methylpiperazin-1-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of CYP2C19 from human liver microsome by LC-MS/MS analysis | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50318596 (3-((4-(6-Bromo-2-(4-(4-methylpiperazin-1-yl)phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of CYP2D6 from human liver microsome by LC-MS/MS analysis | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50318596 (3-((4-(6-Bromo-2-(4-(4-methylpiperazin-1-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of CYP3A4 from human liver microsome by LC-MS/MS analysis | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50318596 (3-((4-(6-Bromo-2-(4-(4-methylpiperazin-1-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human ERG | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50318596 (3-((4-(6-Bromo-2-(4-(4-methylpiperazin-1-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of CYP2C9 from human liver microsome by LC-MS/MS analysis | J Med Chem 53: 5213-28 (2010) Article DOI: 10.1021/jm100262j BindingDB Entry DOI: 10.7270/Q2GB251P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||