

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50321122 2-Ethylquinazolin-4(3H)-one::CHEMBL1163173
SMILES: Clc1ccc(cc1)-c1nc2ccccc2c(=O)[nH]1
InChI Key: InChIKey=AKHUKZJZNGHOJU-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50321122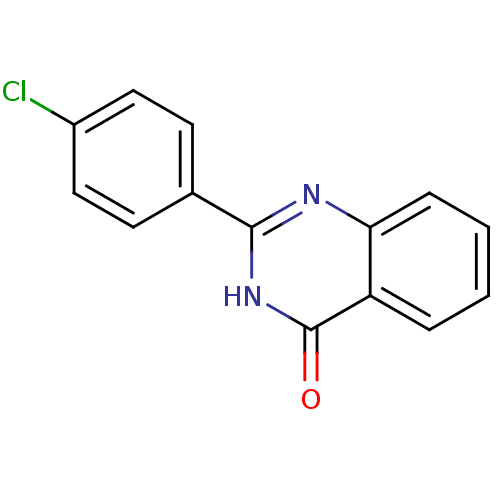 (2-Ethylquinazolin-4(3H)-one | CHEMBL1163173) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Troms£ Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin after 15 mins by microplate fluorescence analysis in presence of 0.5 to 2 mM substrate FaGLa | Bioorg Med Chem 18: 4317-27 (2010) Article DOI: 10.1016/j.bmc.2010.04.083 BindingDB Entry DOI: 10.7270/Q2QV3MP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor (Rattus norvegicus (rat)) | BDBM50321122 (2-Ethylquinazolin-4(3H)-one | CHEMBL1163173) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North-West University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from Sprague-Dawley rat whole brain membrane A1AR by scintillation counting analysis | Bioorg Med Chem Lett 30: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a and A3 (Rattus norvegicus (rat)) | BDBM50321122 (2-Ethylquinazolin-4(3H)-one | CHEMBL1163173) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
North-West University Curated by ChEMBL | Assay Description Displacement of [3H]NECA from Sprague-Dawley rat striatal membrane A2A adenosine receptor by scintillation counting analysis | Bioorg Med Chem Lett 30: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50321122 (2-Ethylquinazolin-4(3H)-one | CHEMBL1163173) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of PARP-1 (unknown origin) using histone as substrate incubated for 1 hr by chemiluminescence assay | Eur J Med Chem 118: 316-27 (2016) BindingDB Entry DOI: 10.7270/Q22F7QC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50321122 (2-Ethylquinazolin-4(3H)-one | CHEMBL1163173) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 485 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of TNKS-1 (unknown origin) using histone as substrate incubated for 30 mins by colorimetric assay | Eur J Med Chem 118: 316-27 (2016) BindingDB Entry DOI: 10.7270/Q22F7QC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50321122 (2-Ethylquinazolin-4(3H)-one | CHEMBL1163173) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of TNKS2 (unknown origin) by fluorescence analysis | ACS Med Chem Lett 6: 1019-24 (2015) Article DOI: 10.1021/acsmedchemlett.5b00251 BindingDB Entry DOI: 10.7270/Q25B05GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50321122 (2-Ethylquinazolin-4(3H)-one | CHEMBL1163173) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of TNKS1 (unknown origin) by fluorescence analysis | ACS Med Chem Lett 6: 1019-24 (2015) Article DOI: 10.1021/acsmedchemlett.5b00251 BindingDB Entry DOI: 10.7270/Q25B05GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50321122 (2-Ethylquinazolin-4(3H)-one | CHEMBL1163173) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human TNKS-2 (849 to 1166 residues) expressed in in insect sf21 cells preincubated for 2 hrs followed by substrat... | Eur J Med Chem 118: 316-27 (2016) BindingDB Entry DOI: 10.7270/Q22F7QC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 2 (Homo sapiens (Human)) | BDBM50321122 (2-Ethylquinazolin-4(3H)-one | CHEMBL1163173) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Inhibition of human PARP-2 (unknown origin) using histone as substrate incubated for 1 hr by chemiluminescence assay | Eur J Med Chem 118: 316-27 (2016) BindingDB Entry DOI: 10.7270/Q22F7QC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| beta-Glucuronidase (β-glucuronidase) (Homo sapiens (Human)) | BDBM50321122 (2-Ethylquinazolin-4(3H)-one | CHEMBL1163173) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of beta-glucuronidase activity (unknown origin) assessed as p-nitrophenol formation after 30 mins using p-nitrophenyl-beta-D-glucuronide a... | Bioorg Med Chem 22: 3449-54 (2014) Article DOI: 10.1016/j.bmc.2014.04.039 BindingDB Entry DOI: 10.7270/Q2057HHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thermolysin (Bacillus thermoproteolyticus) | BDBM50321122 (2-Ethylquinazolin-4(3H)-one | CHEMBL1163173) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Troms£ Curated by ChEMBL | Assay Description Inhibition of Bacillus thermoproteolyticus thermolysin after 15 mins by microplate fluorescence analysis in presence of 0.1 M substrate FaGLa | Bioorg Med Chem 18: 4317-27 (2010) Article DOI: 10.1016/j.bmc.2010.04.083 BindingDB Entry DOI: 10.7270/Q2QV3MP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||