

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50322543 3-[2-(Imidazo[1,2-a]pyridin-3-yl)ethynyl]-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide::CHEMBL1171086
SMILES: CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4ccccn34)cc2C(F)(F)F)CC1
InChI Key: InChIKey=ZRIKPFNXTUUJLL-UHFFFAOYSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50322543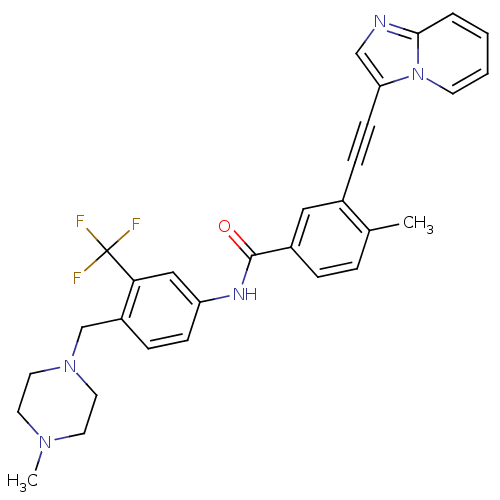 (3-[2-(Imidazo[1,2-a]pyridin-3-yl)ethynyl]-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human ABL after 1 hr by TR-FRET assay | J Med Chem 53: 4701-19 (2010) Article DOI: 10.1021/jm100395q BindingDB Entry DOI: 10.7270/Q27P8ZKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50322543 (3-[2-(Imidazo[1,2-a]pyridin-3-yl)ethynyl]-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of human wild-type Abl after 30 mins by phosphocellulose paper disk assay using 0.1 mM EAIYAAPFAKKK peptide substrate | J Med Chem 58: 9228-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b01125 BindingDB Entry DOI: 10.7270/Q2542RK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50322543 (3-[2-(Imidazo[1,2-a]pyridin-3-yl)ethynyl]-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibition of Abl T315I mutant (unknown origin) after 30 mins by phosphocellulose paper disk assay using 0.1 mM EAIYAAPFAKKK peptide substrate | J Med Chem 58: 9228-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b01125 BindingDB Entry DOI: 10.7270/Q2542RK1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||