

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50322820 CHEMBL1172781::S-2-(5,6-Diphenylfuro[2,3-d]pyrimidin-4-ylamino)-2-phenylethano
SMILES: OC[C@@H](Nc1ncnc2oc(c(-c3ccccc3)c12)-c1ccccc1)c1ccccc1
InChI Key: InChIKey=CCGBAJCQZPJWCS-OAQYLSRUSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50322820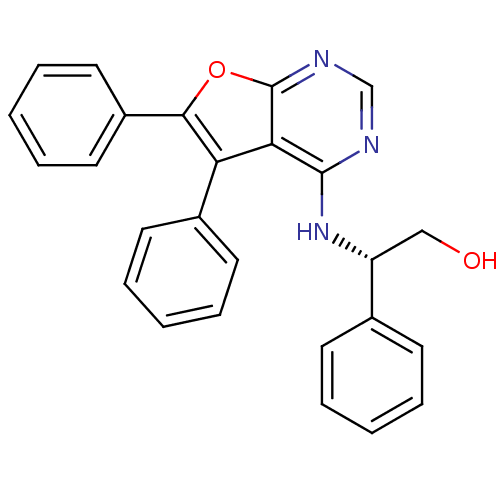 (CHEMBL1172781 | S-2-(5,6-Diphenylfuro[2,3-d]pyrimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged EGFR expressed in Escherichia coli | J Med Chem 53: 4980-8 (2010) Checked by Author Article DOI: 10.1021/jm1000198 BindingDB Entry DOI: 10.7270/Q2Z60P87 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50322820 (CHEMBL1172781 | S-2-(5,6-Diphenylfuro[2,3-d]pyrimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of EGFR | J Med Chem 53: 7316-26 (2010) Article DOI: 10.1021/jm100607r BindingDB Entry DOI: 10.7270/Q26D5T64 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50322820 (CHEMBL1172781 | S-2-(5,6-Diphenylfuro[2,3-d]pyrimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 393 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human wild type GST-tagged EGFR kinase domain expressed in Sf9 cells by luminescence assay | J Med Chem 56: 3889-903 (2013) Article DOI: 10.1021/jm400072p BindingDB Entry DOI: 10.7270/Q27W6DJ5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50322820 (CHEMBL1172781 | S-2-(5,6-Diphenylfuro[2,3-d]pyrimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR kinase domain (696 to 1022 residues) using poly(Glu, Tyr) 4:1 substrate incubated for 60 mins by kinase-Glo plus ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50322820 (CHEMBL1172781 | S-2-(5,6-Diphenylfuro[2,3-d]pyrimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR kinase domain I858R/T790M mutant (696 to 1022 residues) using EGFR L858R/T790M substrate peptide incubated for 12... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50322820 (CHEMBL1172781 | S-2-(5,6-Diphenylfuro[2,3-d]pyrimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR kinase domain (696 to 1022 residues) using poly(Glu, Tyr) 4:1 substrate incubated for 60 mins by kinase-Glo plus ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50322820 (CHEMBL1172781 | S-2-(5,6-Diphenylfuro[2,3-d]pyrimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR kinase domain I858R/T790M mutant (696 to 1022 residues) using EGFR L858R/T790M substrate peptide incubated for 12... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||