

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1c(NC(=O)C(=O)c2ccc(OCCN3CCOCC3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C
InChI Key: InChIKey=UUROSJLZNDSXRF-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scavenger receptor class B member 1 (Mus musculus) | BDBM50323654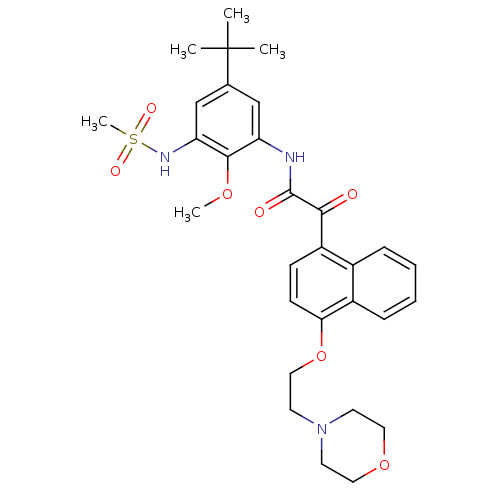 (CHEMBL1208829 | N-(5-tert-butyl-2-methoxy-3-(methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of mouse SR-B1 overexpressed in CHO cells assessed as inhibition of [3H]cholesteryl ester uptake into cells after 2 to 3 hrs by liquid sci... | Bioorg Med Chem Lett 25: 2100-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.073 BindingDB Entry DOI: 10.7270/Q2P84DKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Scavenger receptor class B member 1 (Mus musculus) | BDBM50323654 (CHEMBL1208829 | N-(5-tert-butyl-2-methoxy-3-(methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Marquette University Curated by ChEMBL | Assay Description Inhibition of mouse SR-BI isoform 1 expressed in CHO cells assessed as reduction in uptake of [3H]CE from [3H]CE-HDL by by liquid scintillation count... | Bioorg Med Chem Lett 25: 2594-8 (2015) Article DOI: 10.1016/j.bmcl.2015.03.074 BindingDB Entry DOI: 10.7270/Q25Q4XTV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50323654 (CHEMBL1208829 | N-(5-tert-butyl-2-methoxy-3-(methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc. Curated by ChEMBL | Assay Description Inhibition of phospho-p38 alpha activity by ELISA | Bioorg Med Chem Lett 20: 4819-24 (2010) Article DOI: 10.1016/j.bmcl.2010.06.102 BindingDB Entry DOI: 10.7270/Q2V40W66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50323654 (CHEMBL1208829 | N-(5-tert-butyl-2-methoxy-3-(methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
K£mia, Inc. Curated by ChEMBL | Assay Description Inhibition of p38 alpha activity by ELISA | Bioorg Med Chem Lett 20: 4819-24 (2010) Article DOI: 10.1016/j.bmcl.2010.06.102 BindingDB Entry DOI: 10.7270/Q2V40W66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Scavenger receptor class B member 1 (Homo sapiens (Human)) | BDBM50323654 (CHEMBL1208829 | N-(5-tert-butyl-2-methoxy-3-(methy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human SR-B1 transiently expressed in human U2OS cells assessed as Dil-HDL uptake preincubated for 2 hrs followed by Dil-HDL addition me... | Bioorg Med Chem Lett 26: 1901-4 (2016) BindingDB Entry DOI: 10.7270/Q22R3TJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||