

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50323737 CHEMBL1213687::N-((2S)-1-((2S)-6-amino-1-(4-(6-methoxyquinolin-8-ylamino)pentylamino)-1-oxohexan-2-ylamino)-4-methyl-1-oxopentan-2-yl)-4-(4-((2S,3S)-1-((3S,4S)-1-(butylamino)-3-hydroxy-6-methyl-1-oxoheptan-4-ylamino)-3-methyl-1-oxopentan-2-ylcarbamoyl)phenoxy)benzamide
SMILES: CCCCNC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)c1ccc(Oc2ccc(cc2)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCCCC(C)Nc2cc(OC)cc3cccnc23)cc1)[C@@H](C)CC
InChI Key: InChIKey=JNHJWVLANSQMLI-HTFBEABMSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasmepsin 2 (Plasmodium falciparum) | BDBM50323737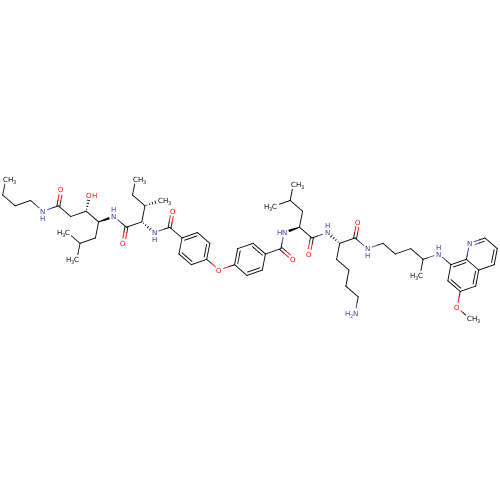 (CHEMBL1213687 | N-((2S)-1-((2S)-6-amino-1-(4-(6-me...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin 2 (Plasmodium falciparum) | BDBM50323737 (CHEMBL1213687 | N-((2S)-1-((2S)-6-amino-1-(4-(6-me...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum plasmepsin 2 | Eur J Med Chem 45: 3245-64 (2010) Article DOI: 10.1016/j.ejmech.2010.04.011 BindingDB Entry DOI: 10.7270/Q26D5T5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||