 Found 10 hits for monomerid = 50324514
Found 10 hits for monomerid = 50324514 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324514
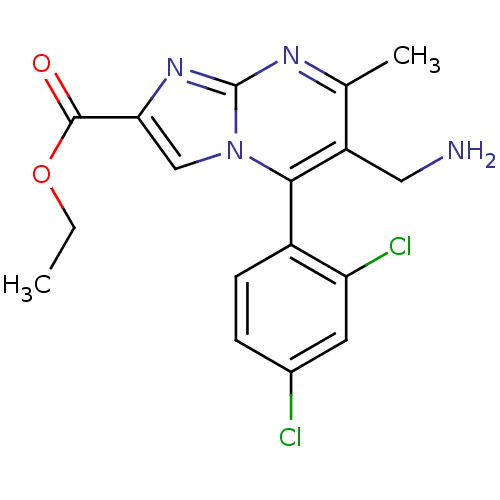
((+)-Ethyl 6-(aminomethyl)-5-(2,4-dichlorophenyl)-7...)Show SMILES CCOC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-9.34,-.68,;-8.61,.68,;-7.07,.72,;-6.34,2.08,;-7.15,3.39,;-4.8,2.13,;-3.86,.91,;-2.41,1.43,;-1.06,.7,;.25,1.51,;1.61,.79,;2.92,1.59,;.2,3.06,;1.51,3.87,;-1.16,3.77,;-2.46,2.96,;-3.93,3.4,;-1.01,-.83,;-2.32,-1.64,;-2.28,-3.18,;-.92,-3.91,;-.87,-5.44,;.4,-3.09,;.34,-1.55,;1.65,-.74,)| Show InChI InChI=1S/C17H16Cl2N4O2/c1-3-25-16(24)14-8-23-15(11-5-4-10(18)6-13(11)19)12(7-20)9(2)21-17(23)22-14/h4-6,8H,3,7,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324514

((+)-Ethyl 6-(aminomethyl)-5-(2,4-dichlorophenyl)-7...)Show SMILES CCOC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-9.34,-.68,;-8.61,.68,;-7.07,.72,;-6.34,2.08,;-7.15,3.39,;-4.8,2.13,;-3.86,.91,;-2.41,1.43,;-1.06,.7,;.25,1.51,;1.61,.79,;2.92,1.59,;.2,3.06,;1.51,3.87,;-1.16,3.77,;-2.46,2.96,;-3.93,3.4,;-1.01,-.83,;-2.32,-1.64,;-2.28,-3.18,;-.92,-3.91,;-.87,-5.44,;.4,-3.09,;.34,-1.55,;1.65,-.74,)| Show InChI InChI=1S/C17H16Cl2N4O2/c1-3-25-16(24)14-8-23-15(11-5-4-10(18)6-13(11)19)12(7-20)9(2)21-17(23)22-14/h4-6,8H,3,7,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324514

((+)-Ethyl 6-(aminomethyl)-5-(2,4-dichlorophenyl)-7...)Show SMILES CCOC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-9.34,-.68,;-8.61,.68,;-7.07,.72,;-6.34,2.08,;-7.15,3.39,;-4.8,2.13,;-3.86,.91,;-2.41,1.43,;-1.06,.7,;.25,1.51,;1.61,.79,;2.92,1.59,;.2,3.06,;1.51,3.87,;-1.16,3.77,;-2.46,2.96,;-3.93,3.4,;-1.01,-.83,;-2.32,-1.64,;-2.28,-3.18,;-.92,-3.91,;-.87,-5.44,;.4,-3.09,;.34,-1.55,;1.65,-.74,)| Show InChI InChI=1S/C17H16Cl2N4O2/c1-3-25-16(24)14-8-23-15(11-5-4-10(18)6-13(11)19)12(7-20)9(2)21-17(23)22-14/h4-6,8H,3,7,20H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324514

((+)-Ethyl 6-(aminomethyl)-5-(2,4-dichlorophenyl)-7...)Show SMILES CCOC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-9.34,-.68,;-8.61,.68,;-7.07,.72,;-6.34,2.08,;-7.15,3.39,;-4.8,2.13,;-3.86,.91,;-2.41,1.43,;-1.06,.7,;.25,1.51,;1.61,.79,;2.92,1.59,;.2,3.06,;1.51,3.87,;-1.16,3.77,;-2.46,2.96,;-3.93,3.4,;-1.01,-.83,;-2.32,-1.64,;-2.28,-3.18,;-.92,-3.91,;-.87,-5.44,;.4,-3.09,;.34,-1.55,;1.65,-.74,)| Show InChI InChI=1S/C17H16Cl2N4O2/c1-3-25-16(24)14-8-23-15(11-5-4-10(18)6-13(11)19)12(7-20)9(2)21-17(23)22-14/h4-6,8H,3,7,20H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324514

((+)-Ethyl 6-(aminomethyl)-5-(2,4-dichlorophenyl)-7...)Show SMILES CCOC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-9.34,-.68,;-8.61,.68,;-7.07,.72,;-6.34,2.08,;-7.15,3.39,;-4.8,2.13,;-3.86,.91,;-2.41,1.43,;-1.06,.7,;.25,1.51,;1.61,.79,;2.92,1.59,;.2,3.06,;1.51,3.87,;-1.16,3.77,;-2.46,2.96,;-3.93,3.4,;-1.01,-.83,;-2.32,-1.64,;-2.28,-3.18,;-.92,-3.91,;-.87,-5.44,;.4,-3.09,;.34,-1.55,;1.65,-.74,)| Show InChI InChI=1S/C17H16Cl2N4O2/c1-3-25-16(24)14-8-23-15(11-5-4-10(18)6-13(11)19)12(7-20)9(2)21-17(23)22-14/h4-6,8H,3,7,20H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324514

((+)-Ethyl 6-(aminomethyl)-5-(2,4-dichlorophenyl)-7...)Show SMILES CCOC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-9.34,-.68,;-8.61,.68,;-7.07,.72,;-6.34,2.08,;-7.15,3.39,;-4.8,2.13,;-3.86,.91,;-2.41,1.43,;-1.06,.7,;.25,1.51,;1.61,.79,;2.92,1.59,;.2,3.06,;1.51,3.87,;-1.16,3.77,;-2.46,2.96,;-3.93,3.4,;-1.01,-.83,;-2.32,-1.64,;-2.28,-3.18,;-.92,-3.91,;-.87,-5.44,;.4,-3.09,;.34,-1.55,;1.65,-.74,)| Show InChI InChI=1S/C17H16Cl2N4O2/c1-3-25-16(24)14-8-23-15(11-5-4-10(18)6-13(11)19)12(7-20)9(2)21-17(23)22-14/h4-6,8H,3,7,20H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324514

((+)-Ethyl 6-(aminomethyl)-5-(2,4-dichlorophenyl)-7...)Show SMILES CCOC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-9.34,-.68,;-8.61,.68,;-7.07,.72,;-6.34,2.08,;-7.15,3.39,;-4.8,2.13,;-3.86,.91,;-2.41,1.43,;-1.06,.7,;.25,1.51,;1.61,.79,;2.92,1.59,;.2,3.06,;1.51,3.87,;-1.16,3.77,;-2.46,2.96,;-3.93,3.4,;-1.01,-.83,;-2.32,-1.64,;-2.28,-3.18,;-.92,-3.91,;-.87,-5.44,;.4,-3.09,;.34,-1.55,;1.65,-.74,)| Show InChI InChI=1S/C17H16Cl2N4O2/c1-3-25-16(24)14-8-23-15(11-5-4-10(18)6-13(11)19)12(7-20)9(2)21-17(23)22-14/h4-6,8H,3,7,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 11.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324514

((+)-Ethyl 6-(aminomethyl)-5-(2,4-dichlorophenyl)-7...)Show SMILES CCOC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-9.34,-.68,;-8.61,.68,;-7.07,.72,;-6.34,2.08,;-7.15,3.39,;-4.8,2.13,;-3.86,.91,;-2.41,1.43,;-1.06,.7,;.25,1.51,;1.61,.79,;2.92,1.59,;.2,3.06,;1.51,3.87,;-1.16,3.77,;-2.46,2.96,;-3.93,3.4,;-1.01,-.83,;-2.32,-1.64,;-2.28,-3.18,;-.92,-3.91,;-.87,-5.44,;.4,-3.09,;.34,-1.55,;1.65,-.74,)| Show InChI InChI=1S/C17H16Cl2N4O2/c1-3-25-16(24)14-8-23-15(11-5-4-10(18)6-13(11)19)12(7-20)9(2)21-17(23)22-14/h4-6,8H,3,7,20H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324514

((+)-Ethyl 6-(aminomethyl)-5-(2,4-dichlorophenyl)-7...)Show SMILES CCOC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-9.34,-.68,;-8.61,.68,;-7.07,.72,;-6.34,2.08,;-7.15,3.39,;-4.8,2.13,;-3.86,.91,;-2.41,1.43,;-1.06,.7,;.25,1.51,;1.61,.79,;2.92,1.59,;.2,3.06,;1.51,3.87,;-1.16,3.77,;-2.46,2.96,;-3.93,3.4,;-1.01,-.83,;-2.32,-1.64,;-2.28,-3.18,;-.92,-3.91,;-.87,-5.44,;.4,-3.09,;.34,-1.55,;1.65,-.74,)| Show InChI InChI=1S/C17H16Cl2N4O2/c1-3-25-16(24)14-8-23-15(11-5-4-10(18)6-13(11)19)12(7-20)9(2)21-17(23)22-14/h4-6,8H,3,7,20H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324514

((+)-Ethyl 6-(aminomethyl)-5-(2,4-dichlorophenyl)-7...)Show SMILES CCOC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-9.34,-.68,;-8.61,.68,;-7.07,.72,;-6.34,2.08,;-7.15,3.39,;-4.8,2.13,;-3.86,.91,;-2.41,1.43,;-1.06,.7,;.25,1.51,;1.61,.79,;2.92,1.59,;.2,3.06,;1.51,3.87,;-1.16,3.77,;-2.46,2.96,;-3.93,3.4,;-1.01,-.83,;-2.32,-1.64,;-2.28,-3.18,;-.92,-3.91,;-.87,-5.44,;.4,-3.09,;.34,-1.55,;1.65,-.74,)| Show InChI InChI=1S/C17H16Cl2N4O2/c1-3-25-16(24)14-8-23-15(11-5-4-10(18)6-13(11)19)12(7-20)9(2)21-17(23)22-14/h4-6,8H,3,7,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
| Assay Description
The HypoGen module in DS2.5 was employed to produce pharmaphores with the training set compounds. |
Chem Biol Drug Des 84: 364-77 (2014)
Article DOI: 10.1111/cbdd.12327
BindingDB Entry DOI: 10.7270/Q2D21W96 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data