

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50324670 ((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl 2-hydroxybenzoylsulfamate::5'-O-(N-(2-hydroxybenzoyl)sulfamoyl)adenosine::5'-O-[N-(salicyl)-sulfamoyl]-adenosine::5'-O-[N-(salicyl)sulfamoyl] adenosine::5'-O-[N-(salicyl)sulfamoyl]adenosine::CHEMBL371502
SMILES: Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COS(=O)(=O)NC(=O)c2ccccc2O)[C@@H](O)[C@H]1O
InChI Key: InChIKey=SABYITLYKSVAAD-CNEMSGBDSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2,3-dihydroxybenzoate-AMP ligase (Mycobacterium tuberculosis) | BDBM50324670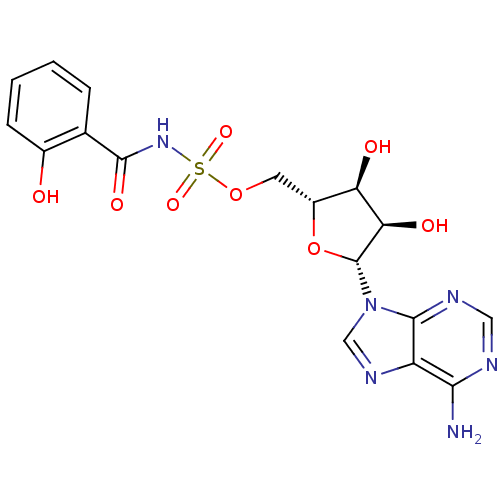 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis MbtA expressed in Escherichia coli assessed as ATP-[32P]Ppi exchange | J Med Chem 51: 7495-507 (2009) Article DOI: 10.1021/jm8008037 BindingDB Entry DOI: 10.7270/Q2HH6M96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilyl-CoA synthetase PqsA (PqsA) (Pseudomonas aeruginosa (Gram- Bacteria)) | BDBM50324670 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Inhibition of recombinant Pseudomonas aeruginosa PqsA assessed as decrease in formation of anthranilyl-CoA by spectrophotometric method | J Med Chem 61: 10385-10402 (2018) Article DOI: 10.1021/acs.jmedchem.8b00540 BindingDB Entry DOI: 10.7270/Q2XP77NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 2 (Homo sapiens (Human)) | BDBM50324670 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Antagonist activity at human TRPM2 expressed in HEK293 cells assessed as inhibition of ADPR-induced maximum outward potassium current at +15 mV by wh... | J Med Chem 56: 10079-102 (2013) Article DOI: 10.1021/jm401497a BindingDB Entry DOI: 10.7270/Q2CZ38P3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 2,3-dihydroxybenzoate-AMP ligase (Mycobacterium tuberculosis) | BDBM50324670 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Medical College of Cornell University Curated by ChEMBL | Assay Description Inhibition of adenylation activity of Mycobacterium tuberculosis MbtA after 30 mins by ATP-[32P]pyrophosphate exchange assay | Nat Chem Biol 1: 29-32 (2006) Article DOI: 10.1038/nchembio706 BindingDB Entry DOI: 10.7270/Q2BV7GV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Yersiniabactin siderophore biosynthetic protein (Yersinia pestis CA88-4125) | BDBM50324670 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Medical College of Cornell University Curated by ChEMBL | Assay Description Inhibition of Yersinia pestis YbtE assessed as incorporation of [3H]salicyl group to HMWP2 protein | Bioorg Med Chem Lett 18: 2662-8 (2008) Article DOI: 10.1016/j.bmcl.2008.03.025 BindingDB Entry DOI: 10.7270/Q2W096TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||