

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50325671 (2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIHYDRO-4H-CHROMEN-4-ONE::(S)-2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-chroman-4-one::(S)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-2,3-dihydrochromen-4-one::(S)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxychroman-4-one::5,7,3',4'-tetrahydroxyflavanone::5,7,3',4'-tetrahydroxyflavon::CHEMBL8996::ERIODICTYOL::eriodicryol::eryodictiol::hsp90_170
SMILES: Oc1cc(O)c2C(=O)C[C@H](Oc2c1)c1ccc(O)c(O)c1
InChI Key: InChIKey=SBHXYTNGIZCORC-ZDUSSCGKSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50325671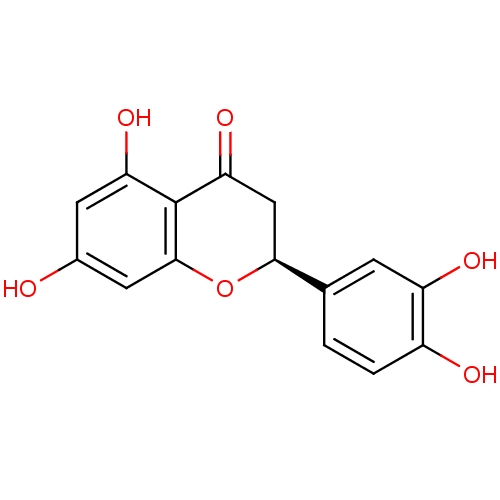 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 4.30 | -11.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 7 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 31 | -10.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration ass... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 73 | -9.73 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 4 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | >1.00E+4 | >-6.82 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | >1.00E+4 | >-6.82 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Aristotle University of Thessaloniki Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins at room temperature/6 hrs at 4 deg C by stopped-flow CO2 hydration assa... | Bioorg Med Chem 23: 7219-25 (2015) BindingDB Entry DOI: 10.7270/Q2862J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.66E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE assessed as acetylthiocholine iodide hydrolysis after 10 mins preincubation by spectrophotometry | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Butyrylcholinesterase (BChE) (Equus caballus (Horse)) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.12E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Gyeongsang National University Curated by ChEMBL | Assay Description Inhibition of equine BChE assessed as butyrylthiocholine iodide hydrolysis after 10 mins preincubation by spectrophotometry | Bioorg Med Chem 20: 2595-602 (2012) Article DOI: 10.1016/j.bmc.2012.02.044 BindingDB Entry DOI: 10.7270/Q22808MC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol hexakisphosphate kinase 2 (Homo sapiens) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Environmental Health Sciences Curated by ChEMBL | Assay Description Inhibition of human IP6K2 using insP6 as substrate preincubated for 15 mins followed by substrate and measured after 30 mins by TR-FRET assay | J Med Chem 62: 1443-1454 (2019) Article DOI: 10.1021/acs.jmedchem.8b01593 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate multikinase (Homo sapiens) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Environmental Health Sciences Curated by ChEMBL | Assay Description Inhibition of human IPMK using insP3 as substrate preincubated for 15 mins followed by substrate and measured after 30 mins by TR-FRET assay | J Med Chem 62: 1443-1454 (2019) Article DOI: 10.1021/acs.jmedchem.8b01593 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1/beta type-5 (Homo sapiens (Human)) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Agricultural and Food Biotechnology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of human 26S proteasome in human Jurkat cells assessed as decrease in AMC hydrolysis using Z-Gly-Gly-Leu-AMC... | Eur J Med Chem 167: 291-311 (2019) Article DOI: 10.1016/j.ejmech.2019.01.044 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1/beta type-5 (Homo sapiens (Human)) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Agricultural and Food Biotechnology Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of purified human 20S proteasome expressed in human Jurkat cells assessed as decrease in AMC hydrolysis usin... | Eur J Med Chem 167: 291-311 (2019) Article DOI: 10.1016/j.ejmech.2019.01.044 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul Curated by ChEMBL | Assay Description Inhibition of recombinant FLT3 (unknown origin) by TR-FRET assay | Bioorg Med Chem Lett 23: 1768-70 (2013) Article DOI: 10.1016/j.bmcl.2013.01.049 BindingDB Entry DOI: 10.7270/Q2H70H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD7 (Homo sapiens (Human)) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human SET7 overexpressed in Escherichia coli BL21 (DE3) cells preincubated for 15 mins followed by addition of SAM as substrate and bio... | Bioorg Med Chem 28: (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 by EROD assay | Bioorg Med Chem 18: 6310-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.020 BindingDB Entry DOI: 10.7270/Q2GB248D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Inhibition of human CYP1A1 by EROD assay | Bioorg Med Chem 18: 6310-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.020 BindingDB Entry DOI: 10.7270/Q2GB248D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Heat shock protein HSP 90-beta (Homo sapiens (Human)) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | D3R | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
D3R | Assay Description FRET Assay 1 | D3R 408: (2016) BindingDB Entry DOI: 10.7270/Q2VX0FC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50325671 ((2S)-2-(3,4-DIHYDROXYPHENYL)-5,7-DIHYDROXY-2,3-DIH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 by EROD assay | Bioorg Med Chem 18: 6310-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.020 BindingDB Entry DOI: 10.7270/Q2GB248D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||