

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50325674 3,5,7-trihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-chromen-4-one::CHEMBL226034::Tamarixetin (22)::tamarixetin
SMILES: COc1ccc(cc1O)-c1oc2cc(O)cc(O)c2c(=O)c1O
InChI Key: InChIKey=FPLMIPQZHHQWHN-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase B (Homo sapiens (Human)) | BDBM50325674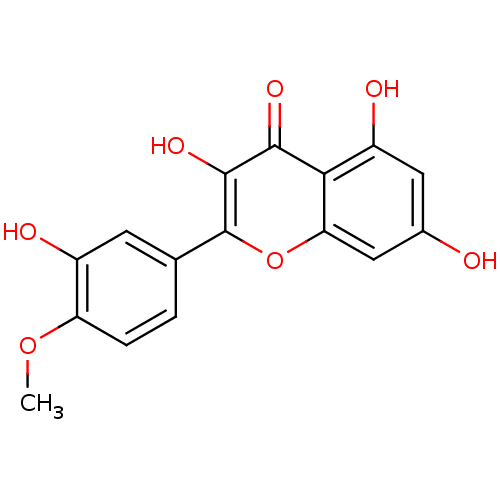 (3,5,7-trihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Konkuk University | Assay Description The kinase assay was performed using the EMD Millipore KinaseProfiler service assay protocol. Aurora B kinase was supplied by EMD Millipore Corp. The... | Chem Biol Drug Des 85: 574-85 (2015) Article DOI: 10.1111/cbdd.12445 BindingDB Entry DOI: 10.7270/Q2M61J08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50325674 (3,5,7-trihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Inhibition of human CYP1A2 by EROD assay | Bioorg Med Chem 18: 6310-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.020 BindingDB Entry DOI: 10.7270/Q2GB248D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (fumarate) (Leishmania major) | BDBM50325674 (3,5,7-trihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant oligo-histidine-tagged Leishmania major DHODH expressed in Escherichia coli BL21(DE3) cells using DHO as substrate measured... | Eur J Med Chem 157: 852-866 (2018) Article DOI: 10.1016/j.ejmech.2018.08.033 BindingDB Entry DOI: 10.7270/Q2FJ2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50325674 (3,5,7-trihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 by EROD assay | Bioorg Med Chem 18: 6310-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.020 BindingDB Entry DOI: 10.7270/Q2GB248D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydroorotate dehydrogenase (fumarate) (Leishmania major) | BDBM50325674 (3,5,7-trihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of S£o Paulo Curated by ChEMBL | Assay Description Inhibition of recombinant oligo-histidine-tagged Leishmania major DHODH expressed in Escherichia coli BL21(DE3) cells using DHO as substrate measured... | Eur J Med Chem 157: 852-866 (2018) Article DOI: 10.1016/j.ejmech.2018.08.033 BindingDB Entry DOI: 10.7270/Q2FJ2KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50325674 (3,5,7-trihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Inhibition of human CYP1A1 by EROD assay | Bioorg Med Chem 18: 6310-5 (2010) Article DOI: 10.1016/j.bmc.2010.07.020 BindingDB Entry DOI: 10.7270/Q2GB248D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||