

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50326557 (E)-5-((2-hydroxynaphthalen-1-yl)diazenyl)naphthalene-2-sulfonic acid::CHEMBL1253920::NSC45576, 2
SMILES: Oc1ccc2ccccc2c1N=Nc1cccc2cc(ccc12)S(O)(=O)=O
InChI Key: InChIKey=COUYJSSQPKTVIQ-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PhoP (Salmonella enterica) | BDBM50326557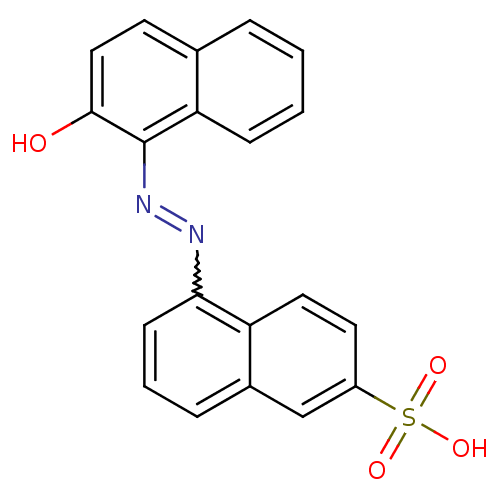 ((E)-5-((2-hydroxynaphthalen-1-yl)diazenyl)naphthal...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine | Assay Description Inhibition of a protein-protein activity by EMSA assay. | Chem Biol Drug Des 79: 1007-17 (2012) Article DOI: 10.1111/j.1747-0285.2012.01362.x BindingDB Entry DOI: 10.7270/Q2FJ2FC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50326557 ((E)-5-((2-hydroxynaphthalen-1-yl)diazenyl)naphthal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Displacement of 8-NBD-cAMP from EPAC2 (unknown origin) by fluorescence plate reader analysis | J Med Chem 57: 3651-65 (2014) Article DOI: 10.1021/jm401425e BindingDB Entry DOI: 10.7270/Q2WH2RJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (PTPβ) (Homo sapiens (Human)) | BDBM50326557 ((E)-5-((2-hydroxynaphthalen-1-yl)diazenyl)naphthal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase B assessed as change in enzyme activity at pH 5 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase alpha (Homo sapiens (Human)) | BDBM50326557 ((E)-5-((2-hydroxynaphthalen-1-yl)diazenyl)naphthal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 5 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase alpha (Homo sapiens (Human)) | BDBM50326557 ((E)-5-((2-hydroxynaphthalen-1-yl)diazenyl)naphthal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||